No Products in the Cart
At the heart of cryogenics, there’s one number that matters more than any other: −196°C (−320°F). This isn't just a reading on a thermometer. It’s the boiling point of liquid nitrogen, the critical temperature that makes groundbreaking science possible and protects everything from life-saving vaccines to priceless biological samples.
Think of the liquid nitrogen temperature not as a fixed number but as a dynamic, powerful force. It is the gold standard for putting biological time on pause, creating an environment so intensely cold that all cellular activity grinds to a halt.
This is the very foundation of modern cryopreservation. When managed with reliable, high-performance equipment, it unlocks incredible possibilities in biotechnology, pharmaceuticals, and advanced manufacturing.
Understanding this deep cold starts with knowing where it fits. Liquid nitrogen sits at a staggering –196°C (–320°F), which easily qualifies it as a cryogenic liquid—a special class of liquefied gases with boiling points below –90°C (–130°F). This property has become indispensable to Germany's world-leading pharmaceutical and biotech sectors. You can read more about the global market drivers for liquid nitrogen to see just how widely its unique properties are applied.
To truly appreciate its role, it helps to see the numbers in context. The liquid state of nitrogen exists in a surprisingly narrow temperature band, which is why precise control is so non-negotiable.
Here is a quick reference table outlining the essential properties that make liquid nitrogen such a versatile tool for scientists and industrial technicians alike.
| Property | Value (Celsius) | Value (Fahrenheit) | Significance |
|---|---|---|---|
| Boiling Point | −196°C | −320°F | The temperature at which liquid turns to gas, defining its operational coldness. |
| Freezing Point | −210°C | −346°F | The point where liquid nitrogen solidifies, a state rarely used in applications. |
This extreme temperature range, from its freezing point at −210°C to its boiling point at −196°C, is precisely what makes it so effective. The constant, gentle boiling at −196°C is what creates the ultra-cold vapour that is absolutely essential for modern biobanking and research.
This isn't just cold; it's a state of suspended animation. The −196°C boiling point creates a stable, ultra-low temperature environment that is predictable and manageable, making it the ideal medium for preserving the building blocks of life.
Ultimately, mastering the liquid nitrogen temperature is all about harnessing this natural phenomenon. When properly contained within expertly engineered cryogenic vessels, this unseen force becomes a predictable and powerful asset. It gives laboratories and industrial facilities the power to secure their most sensitive materials, guaranteeing their integrity and viability for whatever comes next—whether that's groundbreaking research or a life-changing medical treatment.
A cryogenic freezer isn’t just a simple cold box; it’s a surprisingly dynamic thermal environment. The best way to get your head around what's happening inside is to picture a pot of gently boiling water. The heat is at the bottom, creating steam that rises and cools as it reaches the top. A cryogenic vessel is basically the same idea, just flipped upside down.
The liquid nitrogen pools at the bottom, holding a steady temperature right around −196°C. As it inevitably absorbs a tiny bit of heat from the outside world, it boils off, creating an ultra-cold nitrogen vapour. This vapour then fills the space above the liquid, forming a protective, super-chilled atmosphere that is absolutely vital for keeping biological samples safe.
This constant interplay between liquid and gas sets up a natural temperature gradient inside the vessel. This effect, known as temperature stratification, means the environment is far from uniform. It’s coldest at the very bottom where samples are submerged in liquid, and it gets progressively warmer as you move up through the vapour phase toward the lid.
This concept map shows just how central that core -196°C temperature is to everything liquid nitrogen can do.
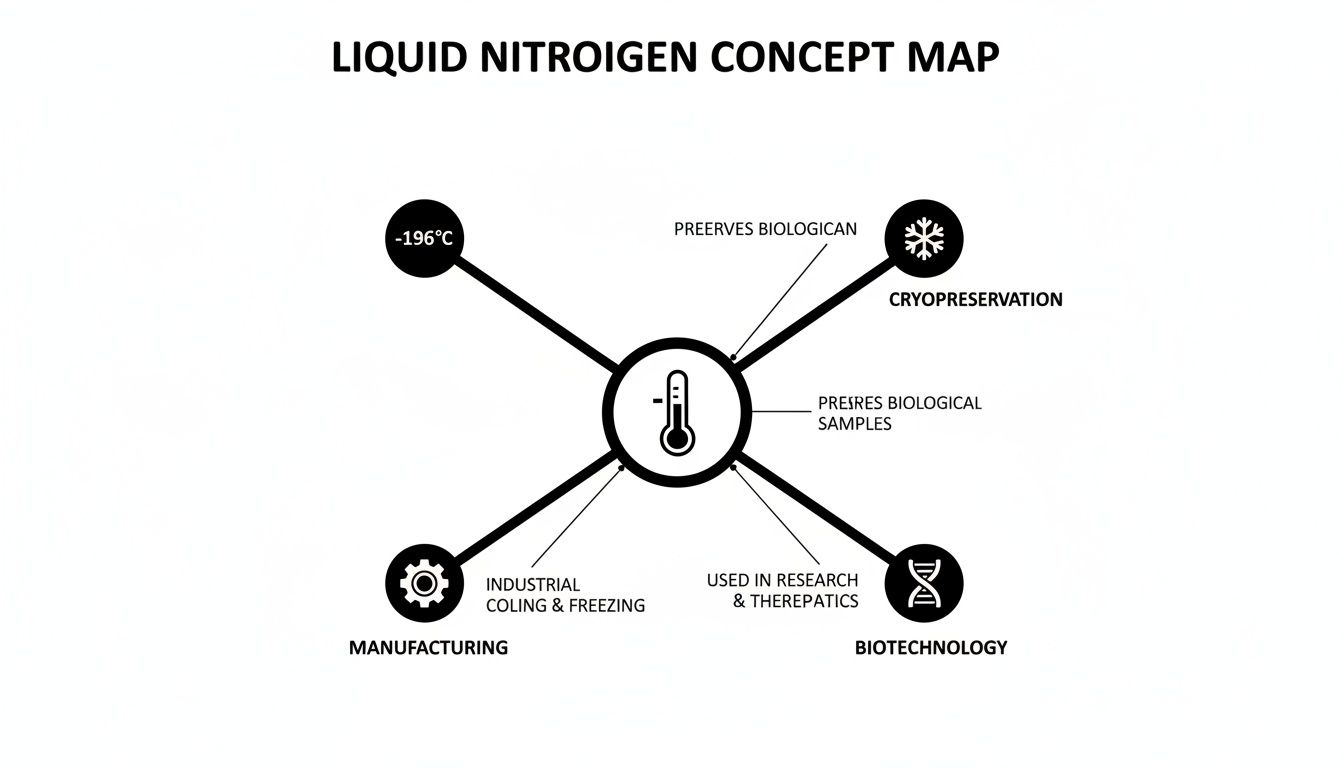
As you can see, that single, critical temperature is the key that unlocks its diverse uses in cryopreservation, biotechnology, and even advanced manufacturing.
The temperature difference from the bottom of a freezer to the top can be surprisingly large, sometimes varying by as much as 20°C to 40°C. For instance, a sample sitting near the bottom might be at a stable −190°C, but one stored near the top could be sitting at a much warmer −150°C.
So, why does this matter so much? Many biological materials have a critical tipping point called the glass transition temperature, which for many cell types is around −135°C. If a sample warms above this temperature, ice crystals can begin to form and grow, effectively shredding delicate cellular structures and ruining the sample’s viability.
The entire goal of cryopreservation is to keep samples safely below this critical threshold. In a poorly managed vessel, it's entirely possible for samples near the top to creep into this danger zone, even when the liquid level at the bottom looks perfectly fine.
This is why knowing where your sample is inside the freezer is every bit as important as knowing the overall liquid nitrogen level.
Several real-world factors can throw off the delicate thermal balance inside a cryogenic vessel. When this happens, you’ll see increased nitrogen consumption and introduce potential risks to your samples. This accelerated evaporation is known as the boil-off rate.
A well-maintained, high-quality vessel is engineered to minimise heat getting in, but things happen. The usual culprits are:
Spotting these issues is the first step to managing them. A sudden buildup of frost around the neck tube or a liquid level that’s dropping faster than usual are tell-tale signs that your vessel's thermal integrity might be compromised.
The boil-off of liquid nitrogen isn't just a nuisance or a sign of inefficiency; it's a fundamental part of how cryogenics works. As the liquid turns into a gas, it absorbs a massive amount of heat energy, and that's precisely what keeps the inside of the vessel cold.
A steady, predictable boil-off rate is perfectly normal. It’s when outside factors accelerate this process that you run into trouble:
Once you understand how the liquid and vapour phases interact and what can disrupt this balance, you can shift from simply using your cryogenic equipment to proactively managing it. This is the key to ensuring peak performance, protecting your invaluable samples, and getting the most out of your operations.
Managing a cryogenic vessel is about more than just keeping it topped up; it’s about knowing precisely what’s happening inside that ultra-cold environment. While liquid nitrogen's temperature is a constant −196°C, the conditions within your freezer certainly are not. This is where accurate measurement and vigilant monitoring become your most important tools for protecting what's inside.
Relying on guesswork or infrequent manual checks is a bit like navigating a ship without a compass. You might think you know your general direction, but you have no way to spot the subtle changes that could lead to disaster. Modern monitoring transforms raw data into actionable insights, giving you the peace of mind that comes from knowing your valuable biological assets are secure.
This whole process hinges on specialised sensors designed to work reliably in the punishing cold of a cryogenic freezer. Without the right tools, you're essentially flying blind.
Not all thermometers are created equal, especially when you're dealing with temperatures plunging toward −200°C. Your standard sensors would simply give up. For cryogenic applications, two types of sensors dominate the field, each with its own set of strengths and ideal use cases.
Here’s a quick look at the most common technologies:
The choice between a thermocouple and an RTD often comes down to balancing your budget against the need for absolute precision. For general monitoring, a Type T thermocouple usually does the job perfectly well. But for applications involving high-value clinical samples or those needing strict GxP compliance, the higher accuracy of an RTD is often worth the investment.
Where you place your sensor is just as critical as the type you choose. As we touched on earlier, temperature stratification means a reading from the top of the freezer tells a very different story than one from the bottom. Sticking a single sensor in the liquid nitrogen will only confirm the obvious: that the liquid is at −196°C.
To get the full picture of your freezer's health, you need multiple measurement points. A sensor at the highest point of your sample storage area is absolutely essential. This location is your early warning system—it's the first place to warm up if the liquid level drops or the lid seal fails.
Effective monitoring strategies almost always involve placing sensors at different heights within the vapour phase. This gives you a clear map of the temperature gradient and helps ensure that all your stored samples remain safely below their critical glass transition temperature. Without this multi-point data, you risk having a false sense of security while samples at the top are creeping toward the danger zone.
Modern monitoring systems do far more than just give you a temperature reading. They provide a continuous, automated record of conditions inside your freezer, which is indispensable for quality control and regulatory compliance. These systems essentially turn your cryogenic freezer into a smart, self-monitoring asset.
Key features of a robust monitoring setup include:
Ultimately, investing in a proper monitoring system is an investment in risk management. It provides the data-driven oversight needed to protect irreplaceable samples, ensure regulatory adherence, and maintain the integrity of your scientific work.
Working with a substance as cold as −196°C demands more than just a little caution; it calls for a deep respect for the physics at play. While the frigid nitrogen liquid temperature is what makes it so incredibly useful, it's also the source of some serious workplace hazards if you don't know what you're doing. Good safety protocols aren't about making people afraid—they're about building confidence and competence.
The real goal is to create a culture where every single action, from topping up a dewar to moving samples, is done with a clear understanding of the risks. This boils down to recognising three main dangers: severe cryogenic burns from contact, asphyxiation if the gas displaces oxygen, and pressure explosions from bad storage habits.

Getting a solid grip on these core risks is the first step toward making the work environment genuinely safe for everyone involved.
The most immediate and obvious danger is getting a cryogenic burn. Any contact with a substance this cold will instantly freeze skin and eye tissue, causing damage very similar to a heat burn. Even a tiny splash can lead to a serious injury, which is why the right Personal Protective Equipment (PPE) is absolutely non-negotiable.
Just as dangerous, but much more subtle, is the risk of asphyxiation. When liquid nitrogen turns back into a gas, it expands massively—one litre of liquid creates nearly 700 litres of nitrogen gas. In a room without good ventilation, this invisible, odourless gas can quickly push out the oxygen.
An oxygen level below 19.5% is considered dangerous. As nitrogen gas fills a space, it quietly drops the amount of available oxygen. This can lead to dizziness, passing out, and eventually death, all without the usual warning signs like feeling short of breath.
To handle these twin threats, every facility needs to enforce strict rules for both personal gear and environmental controls.
Your first and last line of defence against direct exposure is your PPE. A complete set of cryogenic safety gear must be worn every time you handle liquid nitrogen. No excuses.
For a closer look at best practices, check out our guide on the 7 important rules for safe work with cryogenic liquids.
Beyond what you wear, the environment itself has to be set up for safety. Good ventilation is everything. Any area where liquid nitrogen is used or stored needs to have enough air exchange to stop nitrogen gas from building up. Many labs and facilities install permanent oxygen monitors with loud alarms as a critical safety backup.
Proper storage is also key. Liquid nitrogen should only ever be kept in approved, vented containers like Dewars or purpose-built cryogenic freezers.
This is critical: never store liquid nitrogen in a sealed, non-vented container. The liquid is always boiling off, and that gas has to go somewhere. The pressure will build up incredibly fast, creating a massive explosion hazard. It's one of the most dangerous—and easily avoidable—mistakes you can make.
Getting a handle on the nuances of liquid nitrogen temperature is about more than just managing your day-to-day. It’s the key to making a smart, long-term investment in your cryogenic equipment. Concepts like stratification, boil-off, and temperature stability aren't just academic terms; they are the practical realities that should steer every purchase you make, ensuring the gear you pick is a perfect match for what you need it to do.
Choosing the right vessel is always a balancing act between capacity, performance, and its intended job. A massive biobank freezer built for the static, long-haul storage of thousands of samples has a completely different set of engineering priorities than a tough, nimble dry shipper designed to move sensitive materials across the country. One is all about maximising holding time with minimal access, while the other is built for durability and holding its temperature steady on the move.
When you start comparing cryogenic vessels, the spec sheets can look a bit intimidating. But really, only a few key numbers directly impact temperature management and your long-term running costs. The most critical of these is the Static Evaporation Rate, which you'll often see called the boil-off rate.
This figure, usually measured in litres per day, tells you how much liquid nitrogen the vessel will naturally lose over 24 hours under ideal, non-moving conditions. A lower number is always better. It signals superior insulation and more efficient performance.
For instance, a vessel with a boil-off rate of 0.8 litres per day is going to be significantly cheaper to run over its lifetime than a similar-sized model that loses 1.5 litres per day. That difference adds up fast in reduced nitrogen consumption, fewer refills, and less staff time spent topping things up. This is especially important as demand for liquid nitrogen continues to climb. Europe's liquid nitrogen market, valued at around USD 449.2 million in 2025, is projected to hit USD 760.6 million by 2034, signalling some serious growth.
The secret sauce behind a low static evaporation rate is the quality of the vessel's insulation. High-performance cryogenic equipment depends on a sophisticated vacuum system sandwiched between the inner and outer walls. A deeper, more stable vacuum acts as a powerful shield against ambient heat, dramatically slowing the rate at which liquid nitrogen boils away into gas.
Think of it like a premium thermal flask versus a cheap one. Both use the same principle, but the one with the better vacuum will keep your coffee hot (or your drink cold) for hours longer. In a cryogenic vessel, this translates to:
When you choose equipment with advanced vacuum insulation, you aren't just buying a container; you're investing in predictability and peace of mind. You’re ensuring that the nitrogen liquid temperature remains stable, protecting your invaluable assets day in and day out.
Finally, a solid understanding of temperature behaviour helps you focus on the features that truly matter. If your work involves moving samples around frequently, then equipment with ADR-licensed compliance for road transport isn't just a nice-to-have—it’s a must. This certification guarantees the vessel has been engineered to handle the vibrations and temperature swings of transit while keeping its thermal integrity intact.
For biobanks and labs, things like lockable lids and compatibility with advanced monitoring systems become critical for security and regulatory compliance. Even the type of racking system a freezer uses can affect your workflow and temperature stability during sample retrieval. By understanding your daily processes, you can pick a system that minimises air exposure when the vessel is open. Connecting the physics of liquid nitrogen temperature to these practical features is how you make a truly informed decision. Our overview of what to consider when choosing cryogenic vessels offers some extra pointers to help you navigate these choices.
Even when you've got a good handle on the science behind liquid nitrogen's temperature, the practical, day-to-day questions always pop up when you're managing cryogenic equipment. This section dives into some of the most common challenges and queries we hear from researchers, lab managers, and industrial users. Our goal is to give you clear, straightforward answers that tie back to the core concepts we’ve covered, helping you fine-tune your processes and troubleshoot with confidence.
By tackling these real-world scenarios, you can go from simply using liquid nitrogen to truly mastering how it behaves in your specific setup.
One of the first questions people ask is why so many protocols insist on storing biological samples in the vapour phase—the cold nitrogen gas hovering above the liquid—instead of dunking them right in. The answer comes down to one critical factor: eliminating the risk of cross-contamination.
When you submerge samples directly in liquid nitrogen, there's a small but very real risk that microscopic cracks in vials could let the liquid seep inside. If that liquid is carrying pathogens from a leaky vial elsewhere in the tank, you’ve got a contamination event waiting to happen.
Vapour-phase storage, while running at a slightly warmer temperature (typically between −150°C and −190°C), completely removes this liquid-borne contamination path. It creates an incredibly stable and secure environment without the direct contact hazards of liquid immersion.
This trade-off—giving up a few degrees of cold for a huge gain in sample safety—is why vapour-phase storage has become the gold standard for high-value clinical materials, biobanking, and cell therapy. It's the most reliable way to guarantee absolute sample integrity.
Ultimately, choosing the vapour phase is a strategic decision rooted in risk management. It’s about prioritising the purity and safety of irreplaceable biological assets over hitting the absolute lowest temperature possible.
Seeing some ice or frost build up on the outside of your cryogenic vessel, especially around the lid and neck, is a common sight. A little bit of frost is perfectly normal—it’s just moisture from the air freezing as it hits the super-cold surfaces of your equipment.
However, if you're seeing an excessive amount of frost, or it seems to be growing quickly, that’s a clear warning sign. It usually means warm, moist air is leaking into the vessel, and just as importantly, cold nitrogen gas is leaking out. This creates a constant thermal battle that will make your nitrogen consumption soar.
The most frequent culprit? A bad lid seal. If the seal is worn, cracked, or just not sitting right, it creates a perfect pathway for this unwanted heat exchange.
To keep this problem in check and maintain your vessel's efficiency, a few simple habits can make a big difference:
If you’re still seeing heavy, persistent frosting after checking these points, it could point to a more serious issue, like a loss of the vessel's vacuum insulation. At that point, the equipment needs a professional look-over to see if it can be repaired or needs to be replaced.
The temperature of the room where you keep your cryogenic vessel has a direct, measurable impact on how fast you burn through liquid nitrogen. It all comes down to a basic principle of thermodynamics: heat always flows from a warmer area to a colder one. The bigger the temperature difference, the faster that heat moves.
Your cryogenic vessel is built to fight this with a highly effective vacuum layer for insulation. But no insulation is perfect; a small amount of heat will always find its way into the vessel from the surrounding air.
When the room is warm, the temperature gap between the outside air and the −196°C liquid nitrogen inside is much larger. This increased thermal pressure forces heat through the vessel's insulation more quickly.
This extra heat energy gets absorbed by the liquid nitrogen, causing it to boil and turn into gas at a faster rate. This is what we call an increased boil-off rate.
The practical consequences are simple but significant:
Simply storing your vessels in a cooler, well-ventilated, and climate-controlled space can make a real difference. By lowering the ambient temperature, you reduce the thermal load on the vessel. This slows the boil-off rate, saving you both time and money in the long run.
For state-of-the-art cryogenic solutions designed for maximum efficiency and reliability, explore the comprehensive portfolio from Cryonos GmbH. From long-term storage freezers to ADR-licensed transport vessels, we provide the equipment you need to manage the nitrogen liquid temperature with precision and confidence. Visit us at https://www.cryonos.shop to find the right solution for your application.