No Products in the Cart
When people talk about liquid nitrogen, the number that always comes up is -196°C (-320°F). But that's not just a random, frigid number. At standard atmospheric pressure, this is its boiling point—the exact temperature where nitrogen turns from a liquid into a gas. This single physical property is the foundation for its massive role across science and industry.
Thinking about liquid nitrogen’s temperature is less about memorising a figure and more about understanding what’s happening on a physical level. The best way to picture it is to think of a pot of boiling water on your stove. Water hits 100°C (212°F) and violently turns into steam. Liquid nitrogen does the exact same thing, just at the extreme cold end of the thermometer.
At -196°C, it is in a constant state of boiling, continuously releasing incredibly cold nitrogen gas. This constant boiling is precisely what makes it so invaluable. The liquid itself holds a steady, ultra-low temperature, while the gas it throws off creates a frosty, cryogenic environment just above the liquid's surface. This natural process gives us two distinct temperature zones to work with, each with its own advantages.
Inside any cryogenic storage dewar, you’ll find these two states working together, creating a temperature gradient that is crucial for keeping sensitive materials safe.
This distinction is everything in fields like biobanking and cell therapy. The decision to store samples deep in the liquid phase or in the slightly warmer, sterile environment of the vapour phase has massive implications for sample safety and long-term viability.
A great way to visualise this is to imagine a deep, cold lake (the liquid phase) with a thick, icy fog hanging just above its surface (the vapour phase). The lake offers a consistent, deep freeze, while the fog provides a cold buffer zone without any direct liquid contact.
The reason -196°C is such a magic number is that it’s far below the glass transition temperature of water. At this point, all biological activity inside a cell screeches to a halt. Metabolic processes stop completely, but they do so without the formation of large, destructive ice crystals that would otherwise shred the cell from the inside out.
This state of suspended animation is the heart of cryopreservation. It’s what allows us to store everything from human embryos and stem cells to priceless research samples for years, or even decades.
Here's a quick look at the key temperature points:
This table provides a quick summary of the key temperature points and phases of liquid nitrogen relevant for storage and handling.
| Parameter | Temperature (°C) | Temperature (°F) | Key Significance |
|---|---|---|---|
| Boiling Point (Liquid Phase) | -196°C | -320°F | The constant temperature of liquid nitrogen at standard pressure. Maximum coldness and stability. |
| Lower Vapour Phase | ~ -190°C | ~ -310°F | Temperature zone directly above the liquid. Very cold, but eliminates liquid contact risks. |
| Upper Vapour Phase | ~ -150°C | ~ -238°F | Temperature near the top of the dewar. Still cryogenically cold and sufficient for many samples. |
| Glass Transition of Water | ~ -135°C | ~ -211°F | The temperature below which biological activity ceases, preventing ice crystal damage. |
Understanding these different zones is key to effective cryopreservation.
Modern cryogenic systems are engineered specifically to manage these temperature zones with incredible precision. For example, the AC FREEZER series from Cryonos GmbH is designed to provide exceptional temperature uniformity, particularly in the vapour phase. This is vital for applications where preventing any cross-contamination is just as critical as maintaining ultra-low temperatures, ensuring that invaluable biological materials stay viable and secure for the long haul.
When you're setting up a cryogenic storage system, one of the first big decisions you'll face is whether to store your samples in the vapour phase or the liquid phase of nitrogen. This isn't just a minor technical detail; it's a choice that directly impacts sample integrity, safety, and the long-term viability of your entire collection. Both methods rely on the extreme cold of liquid nitrogen, but they go about it in fundamentally different ways.
Think of a cryogenic dewar as a container holding a deep, ultra-cold lake. Storing samples in the liquid phase is like submerging them directly into this lake. This guarantees they are constantly held at the stable boiling point of nitrogen: -196°C (-320°F). This approach offers the most consistent and coldest possible temperature, making it a rock-solid choice for ensuring all metabolic activity comes to a screeching halt.
However, direct immersion isn't without its risks. There's a small but significant chance that if a sample vial isn't perfectly sealed, liquid nitrogen could seep inside. When that vial is retrieved and warms up, the trapped liquid rapidly expands back into a gas, which can cause the vial to rupture explosively. More critically, direct liquid contact creates a potential pathway for cross-contamination, where pathogens could theoretically travel from one compromised sample to another through the shared liquid.
This brings us to the other option: vapour phase storage. Picture this as placing your samples on a secure shelf just above the surface of that icy lake, suspended in the intensely cold "misty fog" that constantly boils off. This vapour isn't one single temperature but exists in a gradient, typically ranging from -190°C right above the liquid line to around -150°C near the top of the freezer.
The biggest win here is the near-total elimination of cross-contamination risk. Since the samples never touch a shared liquid, the pathway for pathogens to spread is completely removed. This has made vapour phase storage the go-to method for high-stakes applications like cell therapy, fertility clinics, and GMP-compliant biobanks, where sample purity is absolutely non-negotiable.
This flowchart shows the distinct states of nitrogen that come into play for cryogenic storage.
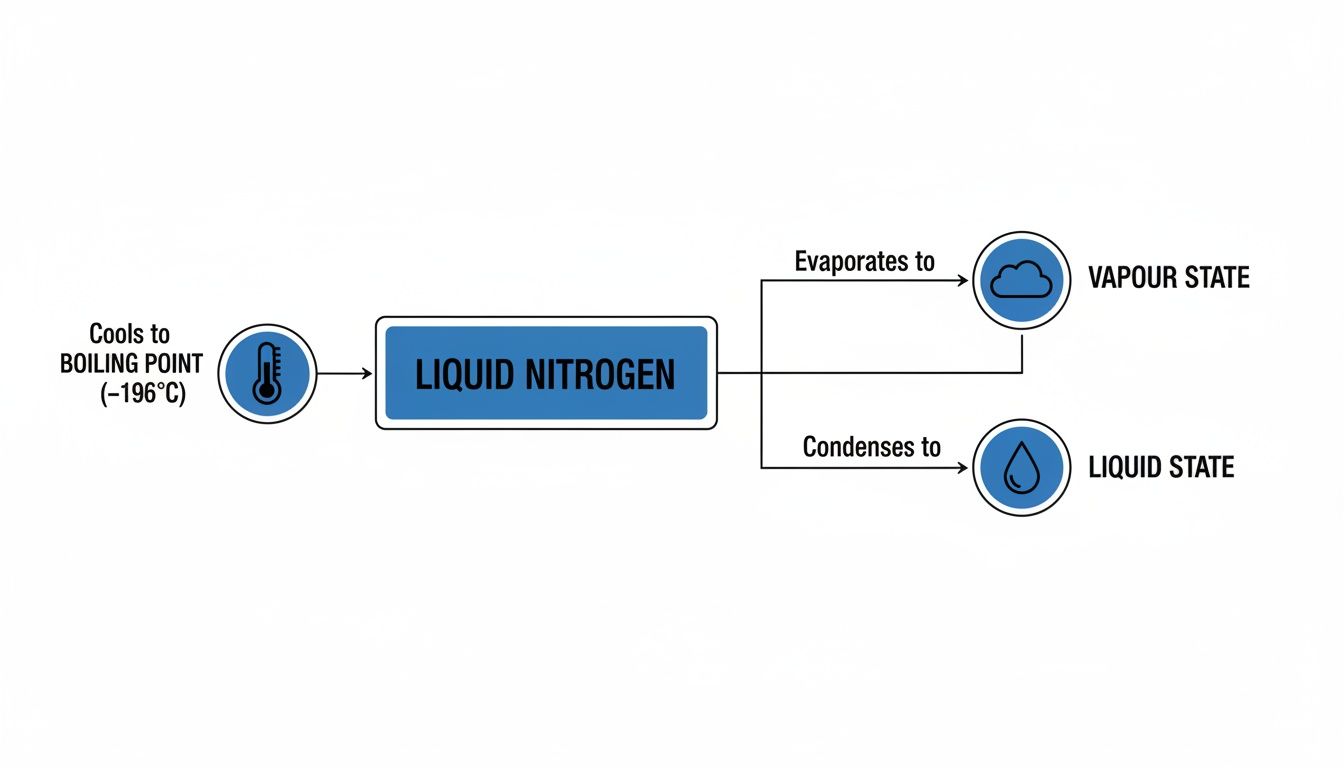
As you can see, the liquid state at -196°C is what generates the cold vapour used for storage, highlighting the crucial physical separation between the two phases.
The trade-off, historically, has been temperature stability. Because the vapour phase is a gradient, keeping a uniformly cold temperature across every single sample rack can be a challenge. A poorly designed freezer might have warmer spots up top, potentially putting samples stored there at risk. This is where the engineering of the storage system itself becomes so incredibly important.
So, which method is right for you? It all comes down to balancing the need for absolute temperature stability against the demand for sample purity and safety. Here’s a quick breakdown to help you decide:
Liquid Phase Storage:
Vapour Phase Storage:
For many modern labs and biobanks, the direction of travel is clear. The shift towards vapour phase storage reflects a growing emphasis on risk mitigation and sample security, especially as regulatory standards rightly become more stringent.
Thankfully, advanced cryogenic systems are now engineered to overcome the old challenges of vapour phase storage. For instance, the Cryonos AC FREEZER series is specifically designed to provide exceptional temperature uniformity across the entire vapour storage area. By optimising internal airflow and insulation, these units ensure that even samples at the very top of the freezer remain safely below the critical glass transition temperature. It’s the best of both worlds: purity and stability.
The temperature of liquid nitrogen, a stark -196°C, isn’t just some random number on the thermometer. It’s the very foundation of modern cryopreservation, a figure that represents the difference between preservation and destruction.
This specific temperature is so effective because it plunges biological samples far below a critical threshold known as the glass transition temperature of water, which sits around -135°C. Hitting this point is like flipping a switch that puts life on pause.
Think of a living cell as a bustling city, teeming with metabolic reactions every second. As you start to cool the cell, all this activity slows down. But once it crosses that glass transition line, everything just… stops. Water inside the cells doesn't form the large, jagged ice crystals that would act like microscopic daggers, shredding delicate cellular structures. Instead, it transforms into a vitrified, glass-like solid.
This state of suspended animation, known as vitrification, is the ultimate goal. It effectively halts all biological activity and decay, locking the sample in a state of perfect preservation. For fertility clinics, biobanks, and cell therapy labs, this translates into incredibly high cell viability rates, often pushing past 95% even after years in storage.
By keeping everything at a steady -196°C, liquid nitrogen provides a crucial safety buffer. This ensures that even with minor, temporary temperature swings—like when a freezer is opened to grab a sample—the core temperature of the stored materials stays safely in that vitrified zone. It's the scientific guarantee behind long-term sample integrity.
The ability to completely stop biological time is fundamental for so many fields. You can dive deeper into its applications in our guide on cryogenic storage for biobanking and stem cell research, which explores how this principle is paving the way for future medical breakthroughs. This deep freeze is what allows scientists to build vast libraries of cells, tissues, and genetic material that remain unchanged for decades.
This preservation power is achieved through a remarkably simple, yet powerful, physical principle.
The constant boiling of liquid nitrogen at -196°C creates a stable, self-regulating environment. As long as there's liquid nitrogen present, the temperature remains locked at this point, providing a reliable deep-freeze that doesn’t depend on complex mechanical refrigeration.
This elegant simplicity makes it an incredibly robust and dependable way to safeguard priceless biological assets.
Of course, operating at the extreme temperature of liquid nitrogen presents some serious engineering challenges. Most everyday materials, like standard plastics and metals, become incredibly brittle at -196°C. Exposing them to that kind of cold can cause them to crack, shrink, or even shatter—a phenomenon called thermal shock. This is why specialised equipment isn't just a good idea; it's absolutely essential.
Cryogenic storage systems must be built from materials that can handle these harsh conditions without failing. This includes:
This meticulous engineering isn't just about storage; it's also about testing and validation. For instance, at its boiling point of -196°C, liquid nitrogen is indispensable for advanced material testing in Germany. Fraunhofer IFAM uses a state-of-the-art cryogenic testing machine to run trials in liquid nitrogen baths, pushing materials to their absolute limits. This kind of capability is vital for clients in biotechnology and pharmaceuticals, ensuring equipment like the AC FREEZER and AC LIN series from Cryonos GmbH can withstand extreme conditions and offer long maintenance intervals. You can learn more about this advanced cryogenic testing on ifam.fraunhofer.de.
Ultimately, the reason -196°C is the gold standard is that it offers a perfect combination of biological effectiveness and physical reliability, making it the bedrock of modern cryopreservation.
Making sure your priceless biological samples stay at the precise temperature liquid nitrogen provides isn't a guessing game. It takes specialised tools, because at -196°C, your standard thermometer is completely useless. Mercury and alcohol would simply freeze solid, turning them into nothing more than delicate glass sticks.
To get reliable data, you need sensors built for the extreme cold. These instruments are the bedrock of any solid cryogenic monitoring strategy, giving you a real-time window into the conditions inside your storage dewar. Without them, you're essentially flying blind—and putting your entire collection at risk.
When it comes to cryogenic applications, two main types of sensors do most of the heavy lifting. Each has its own strengths, and the right choice usually comes down to the precision you need, your budget, and the specific environment where it will be used.
Type T Thermocouples: Think of these as the workhorses of cryogenic monitoring. Made from copper and constantan wires, they're tough, affordable, and dependable across a huge temperature range, all the way down to liquid nitrogen levels. Their biggest advantages are durability and cost-effectiveness, especially if you need to monitor multiple points.
Platinum Resistance Thermometers (RTDs): When absolute precision is the top priority, RTDs are the way to go. They work by measuring the change in electrical resistance of a tiny platinum element. This gives them far greater accuracy and stability than thermocouples, but they tend to be more expensive and a bit more fragile.
For most biobanking and laboratory work, a high-quality Type T thermocouple strikes the perfect balance between accuracy, reliability, and cost. It’s more than capable of catching the small temperature shifts that could signal a problem.
Where you put the sensor is just as critical as which type you choose. A poorly placed sensor can feed you misleading readings, creating a false sense of security while your samples are actually in danger. Smart placement is key to getting a true picture of the conditions throughout the dewar.
For truly comprehensive monitoring, it's best to use multiple sensors to keep an eye on both the liquid and vapour phases.
This dual-sensor strategy gives you a complete view of the dewar's temperature gradient from top to bottom.
Checking temperatures by hand is time-consuming and leaves the door open to human error. Modern inventory management and monitoring systems take this entire process off your plate, providing a powerful safety net for your valuable assets. These systems are non-negotiable for maintaining regulatory compliance and guaranteeing sample integrity.
Automated solutions offer continuous, round-the-clock surveillance. Advanced tools like the AC Data Logger for Dry Shipper series provide real-time data logging, which is crucial for transport and storage validation. If the temperature ever strays from the safe zone, the system instantly shoots out alerts via text or email, letting your team jump in and fix the problem before any damage is done.
For cell therapy labs, the precision of liquid nitrogen at -196°C allows for sub-zero storage with impressive 99% sample recovery rates. Cryonos supports this high standard with its AC Micro Bulk and cell transport units, which provide medical-licensed quality. In Germany, this level of precision allows pharmaceutical firms to deploy -196°C reactors with ±0.5°C control, cutting reaction times by 30%. By pairing the right sensors with automated monitoring, labs can proactively protect their samples and maintain perfect data logs for audits and quality control.

Working with a substance as intensely cold as liquid nitrogen demands more than just a bit of caution—it requires a genuine respect for the risks involved. A simple splash, a poorly ventilated room, or a tightly sealed container can escalate a routine task into a serious incident. To build a truly safe work environment, you first have to understand the 'why' behind the rules.
The dangers of liquid nitrogen aren't just about the cold. While its -196°C temperature can cause severe cryogenic burns in an instant, two invisible hazards are just as threatening. As it warms up, every litre of liquid nitrogen expands into nearly 700 litres of nitrogen gas, creating a massive asphyxiation risk. If that expansion happens inside a sealed container, the pressure build-up can be enormous, leading to a violent explosion.
These three core hazards—cryogenic burns, asphyxiation, and pressure explosions—are the bedrock of all safety protocols. Adhering to these is a non-negotiable part of any comprehensive occupational health and safety management system, providing a structured, reliable approach to controlling the risks.
When you're handling liquid nitrogen, your standard lab gear just won't cut it. Latex gloves or basic safety glasses are dangerously inadequate; they will freeze, become brittle, and shatter on contact, offering you zero protection. Proper Personal Protective Equipment (PPE) is your first and most critical line of defence.
Your mandatory safety gear should always include:
This level of protection isn't just a suggestion—it's absolutely mandatory for any task involving open containers of liquid nitrogen.
Perhaps the most underestimated danger of liquid nitrogen is its ability to silently displace oxygen. As the liquid evaporates into a colourless, odourless gas, it can reduce the oxygen concentration in a room to dangerously low levels without anyone noticing. This makes proper ventilation an absolute necessity, not just a recommendation.
You should always be working in a well-ventilated area. For smaller rooms or enclosed spaces where you're handling larger quantities, mechanical ventilation and continuous monitoring become critical.
Installing an oxygen monitor is one of the most effective safety measures you can take. These devices constantly sample the air and will sound a loud alarm if the oxygen level drops below a safe threshold, typically 19.5%. This gives personnel the crucial time they need to evacuate.
This kind of proactive monitoring turns an invisible threat into a manageable one.
The final piece of the safety puzzle is all about how you handle and store liquid nitrogen. Because of the immense pressure generated during evaporation, you must never store it in a completely sealed container. All approved storage vessels, known as dewars, are engineered with pressure-relief mechanisms that vent this excess gas safely.
Liquid nitrogen, with its ultra-low temperature of -196°C at atmospheric pressure, is a cornerstone of Germany's cryogenic industry. Safety is paramount, and it's paying off: incidents dropped by 25% in Germany from 2018-2023, thanks to enhanced protocols and equipment like those from Cryonos. This track record positions Cryonos as a turn-key supplier for securely handling sensitive materials, serving everyone from university researchers to biotech firms that need reliable nitrogen storage.
Always remember to pour liquid nitrogen slowly and carefully to minimise splashing and rapid boiling. Following these established procedures, from wearing the right gear to ensuring proper ventilation, is fundamental. For a more detailed breakdown, you might find our guide on the 7 important rules for safe work with cryogenic liquids helpful for day-to-day operations.
Even when you've got the basics down, working with liquid nitrogen day in and day out throws up its own set of practical questions. Let's tackle some of the most common ones that pop up in labs, biobanks, and industrial settings to clear up any confusion around safety and handling.
That magic number you always hear—-196°C (-320°F)—is a constant, but it's pinned to standard atmospheric pressure at sea level. If you were to increase the ambient pressure, the boiling point would actually nudge up a bit. Decrease the pressure, and it drops.
But here’s the reality: for virtually every application you'll encounter, from bio-storage to industrial freezing, these tiny pressure variations don't matter. You can absolutely count on -196°C as the reliable, universal reference temperature for all your work.
Yes, and in some ways, it's even more deceptive. The vapour is still intensely cold, and direct contact can cause severe cryogenic burns to skin and eyes, just like a splash from the liquid itself.
The bigger, more insidious threat, however, is asphyxiation. When liquid nitrogen boils, it expands into an enormous cloud of nitrogen gas, ballooning to nearly 700 times its liquid volume. This gas is invisible, has no smell, and pushes all the oxygen out of the air. In a poorly ventilated room or a confined space, oxygen levels can plummet to deadly lows in moments. Always, always ensure there is powerful ventilation.
The liquid burns on contact, but the vapour can create a life-threatening environment. Both phases of liquid nitrogen demand the highest level of respect and adherence to safety protocols. Treat the invisible gas with the same caution as the visible liquid.
Absolutely not. This is a non-negotiable safety rule. You must only use a purpose-built, non-pressurised container called a dewar flask for storing or moving liquid nitrogen, no matter how small the amount.
Think about it: a regular thermos is designed to be a perfectly sealed container. As the liquid nitrogen inside inevitably boils, it generates a huge amount of gas pressure. With nowhere to go, that pressure will build and build until the thermos fails catastrophically—essentially turning it into a bomb. Dewars are smartly engineered with pressure-relief systems that safely vent this gas, preventing a dangerous explosion.
For state-of-the-art cryogenic solutions engineered for maximum safety and performance, explore the complete portfolio at Cryonos GmbH. From advanced storage freezers to ADR-licensed transport vessels, find reliable equipment for your needs at https://www.cryonos.shop.