No Products in the Cart
When you think about the temperature of nitrogen, one number stands out above all others: -195.8°C (-320.4°F), or 77.4 Kelvin. This isn't just a random figure from a science textbook. It's the boiling point of liquid nitrogen at standard atmospheric pressure, and it's the very foundation upon which countless cryogenic applications are built.
The extreme cold of liquid nitrogen is more than just a measurement; it’s a state of being that allows us to do incredible things. It slows down or completely halts molecular and cellular activity, creating what is essentially nature's ultimate stasis field. This unique capability is the cornerstone for biobanks, pharmaceutical labs, and fertility clinics—all of which depend on cryogenic storage to protect priceless biological samples.
However, nitrogen's temperature isn't a single, fixed number. It's dynamic, changing with its physical state—solid, liquid, or gas—and the pressure around it. This relationship is absolutely critical for managing cryogenic vessels safely and effectively. For example, as liquid nitrogen boils off inside a sealed dewar, it transforms into gas. This process builds up internal pressure, which in turn slightly raises the boiling point of the remaining liquid.
For anyone working with cryogenic systems, truly understanding the nuances of nitrogen's temperature is non-negotiable. Here’s why it matters so much:

To make things easier, we've put together a simple table outlining the key temperature points for nitrogen. Think of this as your go-to guide for the numbers that matter most in day-to-day cryogenic work.
Nitrogen Temperature Quick Reference Guide
| State or Point | Temperature in Celsius | Temperature in Kelvin | Practical Significance |
|---|---|---|---|
| Boiling Point | -195.8°C | 77.4 K | The standard temperature of liquid nitrogen used for storage and transport. |
| Melting Point | -210°C | 63.2 K | The point at which solid nitrogen turns into a liquid. |
| Triple Point | -210.01°C | 63.15 K | The unique condition where solid, liquid, and gas phases coexist in equilibrium. |
| Critical Point | -147°C | 126.2 K | Above this temperature, nitrogen can no longer be liquefied, regardless of pressure. |
These values provide the essential framework for understanding how nitrogen behaves under different conditions, helping you maintain optimal performance and safety in your lab or facility.
The consistent, ultra-low temperature of liquid nitrogen provides a reliable environment for long-term preservation. This stability is the key to successful cryopreservation, ensuring that biological samples remain viable for decades.
Ultimately, getting a solid grasp of nitrogen's temperature behaviour is the first step towards mastering cryogenic management. It empowers researchers and technicians to protect sensitive materials and run their processes safely and smoothly. The technology built around this fundamental property has unlocked countless advancements, from preserving life-saving stem cells to enabling modern manufacturing. You can explore more of these fascinating uses in our detailed guide on diverse cryogenic applications.
While most people know liquid nitrogen for its boiling point, that's just one chapter in a much bigger story. To really get a handle on the temperatur von stickstoff, we need to look at the different physical states it can take on. Each state—solid, liquid, and gas—is marked by a specific temperature threshold. These points aren't just academic trivia; they're the core principles behind designing and safely operating any cryogenic system.
Think of these temperatures as milestones on a thermodynamic road trip. As nitrogen loses energy and gets colder, it transitions from a gas to a liquid. If we keep pulling heat away, it eventually turns solid. Understanding these shifts is the key to predicting how nitrogen will behave and keeping stored samples safe.
We all know about boiling and freezing points from our daily experience with water. Nitrogen has them too, just at much more extreme temperatures. These two points define the liquid state we work with at standard atmospheric pressure.
Boiling Point (-195.8°C or 77.4 K): This is the temperature where liquid nitrogen energetically turns into gas. It's the standard operating temperature you'll find inside a non-pressurised cryogenic dewar, where that constant, gentle boiling is completely normal.
Melting/Freezing Point (-210°C or 63.2 K): At this incredibly cold temperature, liquid nitrogen freezes into a clear, crystalline solid that looks a bit like ice. While solid nitrogen has some very specific uses in research, you’ll almost never see it in typical cryogenic storage setups.
These two points create the temperature window where nitrogen stays a liquid—the state that’s so valuable for biobanks, labs, and industrial work. The stability within this range is what makes reliable cryopreservation possible.
Now for a more unusual idea: the triple point. Picture a very specific, unique moment where a substance can be a solid, a liquid, and a gas all at the same time, in perfect equilibrium. For nitrogen, this magic moment happens at a precise temperature of -210.01°C (63.15 K) and a very low pressure of about 0.125 atmospheres.
At its triple point, solid nitrogen, liquid nitrogen, and nitrogen vapour are all in balance. This means all three phases can coexist without one taking over. It’s not a condition you'd find outside of a controlled lab, but the triple point is a critical benchmark in thermodynamics. It acts as a fixed reference point for calibrating scientific instruments with incredible accuracy.
Understanding these thermodynamic points isn't just about theory. It’s about building safer, more efficient cryogenic equipment. Every Cryonos vessel is engineered with these physical laws in mind to ensure maximum performance and sample security.
The triple point really shows the delicate dance between temperature and pressure. Even the tiniest change to either one would knock the nitrogen out of equilibrium, forcing it into a single state. This precision underscores just how vital stable conditions are for cryogenic storage.
Finally, we get to the critical point, which you can think of as the point of no return for turning nitrogen gas back into a liquid. For nitrogen, the critical point is hit at a temperature of -147°C (126.2 K) and a pressure of roughly 33.5 atmospheres.
What's so special about this? Well, once nitrogen gets hotter than this temperature, no amount of pressure—no matter how high—can force it back into a liquid state. Instead, it enters a phase called a supercritical fluid. This state is a fascinating hybrid; it's dense like a liquid but flows and spreads like a gas, letting it penetrate materials in a way a liquid can't.
This has direct, practical implications for cryogenic storage safety. If a sealed container of liquid nitrogen were to warm up past -147°C, the pressure inside would skyrocket.
Each of these key temperatures—boiling, freezing, triple, and critical—defines nitrogen’s physical behaviour and operational limits. Knowing the temperatur von stickstoff at these key junctures allows engineers to design robust cryogenic vessels and helps technicians handle liquid nitrogen with the safety and respect it demands. It’s this deep understanding of physics that ensures the reliability of every Cryonos storage solution.
When you're working with liquid nitrogen, temperature and pressure are two sides of the same coin. They have an inseparable, predictable relationship that’s absolutely critical for safe handling and storage. This connection isn't just a scientific curiosity; it's the core principle behind the design of every single high-quality cryogenic vessel.
Think about boiling a kettle. As the water heats up, it turns into steam. If you trapped that steam, the pressure inside the kettle would skyrocket. Liquid nitrogen behaves in much the same way, just at mind-bogglingly low temperatures. Even inside the best-insulated dewar, a tiny bit of ambient heat will always sneak in, causing the liquid nitrogen to boil constantly.
This slow, continuous evaporation is known as boil-off, and it generates nitrogen gas. In a sealed container, that gas has nowhere to escape, so the internal pressure starts to climb. As pressure builds, it pushes down on the liquid's surface, making it harder for molecules to break free and turn into gas. This means more energy—and therefore a higher temperature—is needed to keep the liquid boiling.
In short, increasing the pressure raises the boiling point of nitrogen.
Getting a handle on this concept is vital for anyone in cryogenics. The clearest way to see this interplay is with a phase diagram, which maps out the different states of a substance (solid, liquid, gas) under various conditions. It shows you exactly how the temperatur von stickstoff is tied to the pressure it's under.
This relationship is precisely why pressure build-up inside a sealed cryogenic tank is so dangerous. Without a way to vent the accumulating gas, the pressure can rise to catastrophic levels. That's why every professionally engineered cryogenic vessel is equipped with safety relief valves—they are a non-negotiable safety feature. These valves are designed to automatically release pressure long before it reaches a hazardous point, protecting both the vessel and, more importantly, the people around it.
You can learn more about how pressure influences other physical properties, like the density of a gas, in our related article.
The chart below lays out the key temperature points for nitrogen at standard atmospheric pressure, offering a simple reference for its different physical states.
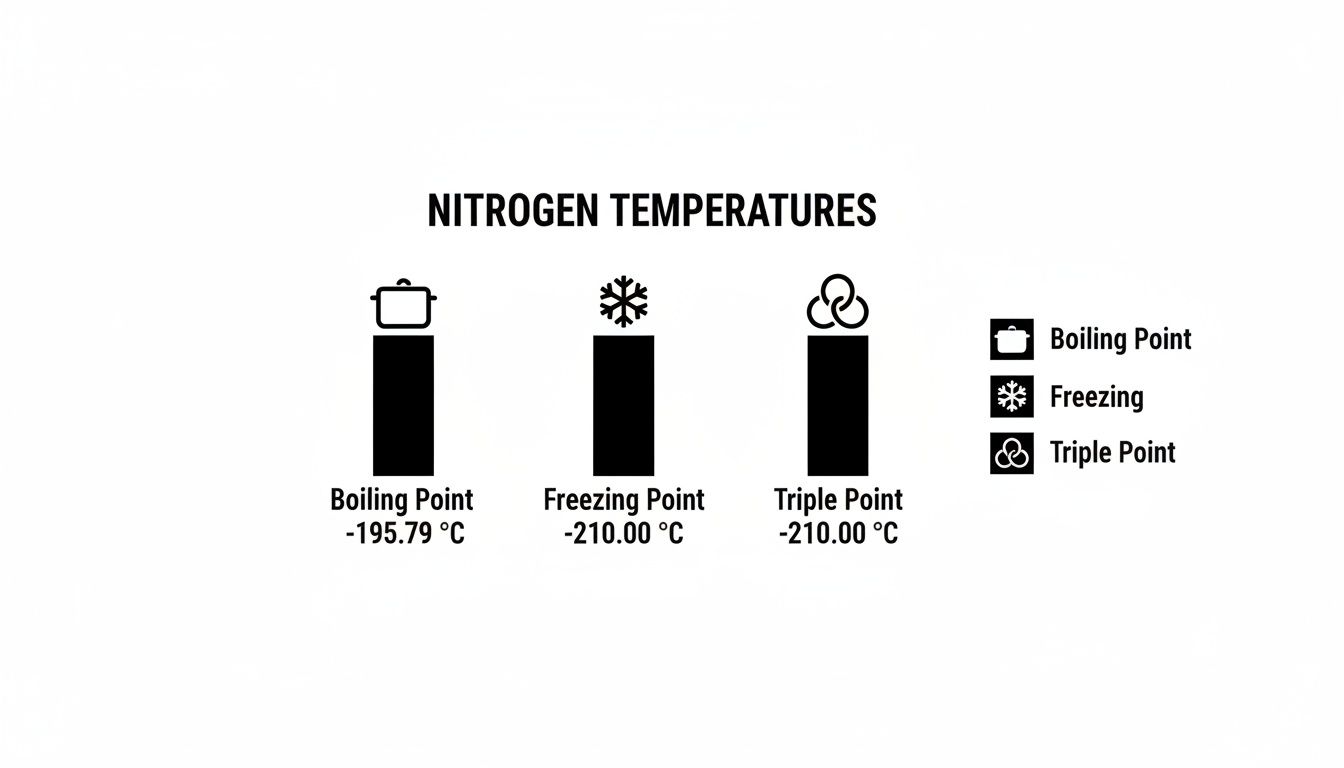
This visual guide highlights the specific temperatures that mark nitrogen's transitions between solid, liquid, and gas—the foundational knowledge for all cryogenic work.
Since heat getting in is the root cause of both boil-off and pressure build-up, the quality of a vessel's insulation is everything. Superior cryogenic equipment, like the solutions we offer at Cryonos, uses a high-vacuum layer sandwiched between the inner and outer walls to drastically cut down on heat ingress.
The goal of advanced insulation is to slow down the inevitable. By minimising heat transfer, we reduce the rate of evaporation, which in turn slows the pace of pressure increase. This extends holding times and enhances the overall safety and efficiency of the storage system.
This level of engineering is what makes reliable, long-term cryogenic storage possible. It’s the science that keeps your valuable samples safely preserved under stable conditions.
Ultimately, mastering the delicate balance between the temperatur von stickstoff and pressure is a constant in cryogenic work. By understanding this relationship and relying on expertly engineered equipment designed to manage it, you can ensure your operations are both safe and successful.
Knowing the physics behind nitrogen is one thing, but using that knowledge to keep priceless biological samples safe? That's where the real challenge lies. For biobanks, fertility clinics, and research labs, mastering the precise control of the temperatur von stickstoff isn't just a technical exercise—it’s the bedrock of their entire operation. The future of every cell, tissue, or sample hinges on maintaining an absolutely stable, ultra-low temperature.
This brings us to a critical concept: the glass transition temperature. For most biological samples, this threshold is around -135°C. If the temperature rises above this point, water molecules can form microscopic ice crystals. Think of them as tiny, sharp daggers that can pierce cell membranes, destroying the sample's integrity from the inside out. By keeping everything consistently colder than this, all cellular activity is effectively frozen in time in a stable, glass-like (vitrified) state, preserving it for years or even decades.
Liquid nitrogen, the cornerstone of Cryonos GmbH's cryogenic storage and transport solutions, boils at a steady -195.8°C under normal atmospheric pressure. This physical constant is pivotal for Germany's leading biobanks and cell therapy labs. It offers a generous safety margin well below the glass transition point, cementing its status as the gold standard for long-term cryopreservation. You can dive deeper into these vital applications in advanced cell research.

Even inside a state-of-the-art cryogenic freezer, the temperature isn't perfectly uniform from top to bottom. This effect, known as temperature stratification, creates distinct temperature zones within a single storage tank. Getting a handle on these zones is essential for optimising sample safety and picking the right storage method.
There are two main ways to store samples:
The primary puzzle with vapour phase storage is managing the temperature gradient. It's coldest right above the liquid and gets progressively warmer toward the lid. A top-tier cryogenic freezer is specifically engineered to keep this gradient to a minimum, ensuring even the samples on the highest rack stay safely below -150°C.
The choice between liquid and vapour phase storage comes down to a risk-benefit analysis. While the liquid phase guarantees absolute temperature stability, the vapour phase provides unmatched protection against cross-contamination—a non-negotiable priority in clinical and pharmaceutical work.
You simply can't manage what you don't measure. In cryogenics, that's an unbreakable rule. Popping in for occasional temperature checks just doesn't cut it when you're guarding irreplaceable biological assets. A short temperature spike—maybe from a lid left open a bit too long or a dip in the liquid nitrogen level—can easily go unnoticed and cause permanent damage.
This is why continuous, automated monitoring systems are non-negotiable in any modern cryogenic facility. These systems are the vigilant sentinels, providing a constant stream of data to ensure the temperatur von stickstoff stays exactly where it needs to be.
Here’s what continuous monitoring brings to the table:
At the end of the day, the reliability of your cryogenic equipment is the last line of defence for your samples. From the vacuum insulation that slows boil-off to the advanced monitoring systems standing guard 24/7, every part has to work in concert to create a secure, stable home for your life's work. It's this level of engineering that gives researchers and clinicians total peace of mind.
Working with the extreme temperatur von stickstoff isn’t just another scientific task—it's a serious responsibility. While incredibly useful, the immense cold of liquid nitrogen presents significant hazards if you don't handle it with the utmost care and respect. A rigorous commitment to safety is absolutely non-negotiable for protecting everyone from harm and maintaining a secure lab environment.
The biggest dangers fall into three main categories: severe cold burns, asphyxiation, and over-pressurisation. Getting a solid grasp of these risks is the first step toward managing them. Each one requires specific protocols and the right gear to turn a potential disaster into a controlled, manageable procedure.

Direct contact with liquid nitrogen or surfaces it has cooled can cause severe tissue damage, much like a thermal burn. In just seconds, skin can become brittle and freeze solid. This is exactly why a complete set of Personal Protective Equipment (PPE) is your first and most important line of defence.
Your essential PPE checklist must include:
A quick but critical note: standard lab gloves, like latex or nitrile, offer zero protection against the extreme cold of liquid nitrogen. They can freeze instantly and may even fuse to your skin, making a bad situation much worse.
When liquid nitrogen evaporates, it expands into a colourless, odourless gas at an astonishing ratio of roughly 1 to 694. To put that in perspective, one litre of liquid nitrogen will produce nearly 700 litres of nitrogen gas. In a poorly ventilated space, this rapidly expanding gas displaces oxygen.
The environment becomes hazardous once oxygen levels in the air drop below 19.5%. Because nitrogen gas is completely undetectable by human senses, a person can become disoriented, lose consciousness, and suffocate without any warning at all. This silent danger is what makes proper ventilation and monitoring so critical.
To combat this risk, you must have the right engineering controls in place:
That massive expansion from liquid to gas creates another serious hazard: over-pressurisation. If liquid nitrogen is trapped in a sealed container with no way for the expanding gas to escape, the internal pressure can build to catastrophic levels. The result? The container can rupture or even explode with tremendous force.
This is why you should never store liquid nitrogen in any container that can be tightly sealed. All proper cryogenic vessels, like the dewars we provide, are specifically designed with pressure-relief mechanisms. These built-in safety devices are engineered to automatically vent excess gas, keeping the internal pressure well within a safe range.
For a deeper dive into day-to-day operational safety, you might find our guide on the 7 important rules for safe work with cryogenic liquids helpful. Following these guidelines ensures that the powerful properties of liquid nitrogen remain a tool for innovation, not a source of danger.
When you work with liquid nitrogen every day, you start to get a feel for its quirks. Here are a few of the most common questions we hear, broken down into simple, practical answers.
It’s all about the massive temperature difference. Liquid nitrogen has a boiling point of a mind-bogglingly cold -195.8°C, whereas a typical room is a balmy 20°C. To the liquid nitrogen, everything around it—the air, the container, you—is like a blast furnace.
This constant bombardment of heat energy forces the liquid to absorb it and rapidly convert into gas, which we see as intense boiling. It’s precisely why you need a high-quality, vacuum-insulated vessel; you're not just holding the cold in, you're fighting to keep the heat out.
Absolutely. In fact, storing your biological samples in the vapour phase just above the liquid nitrogen is often the gold standard, especially in clinical and pharmaceutical labs. Why? It practically eliminates the risk of cross-contamination that can sometimes happen when samples are submerged directly in the liquid.
The temperature in the vapour phase stays incredibly stable and cryogenically cold, usually somewhere between -150°C and -190°C.
The real secret to successful vapour-phase storage is a top-notch freezer. A well-engineered unit will minimise temperature gradients from top to bottom, keeping the entire storage space safely below the critical glass transition temperature of about -135°C.
This gives you peace of mind that every last sample, even the ones at the very top of the rack, is perfectly preserved and protected from cellular damage.
This is a great question that often gets overlooked. Altitude directly impacts the boiling point of any liquid, and nitrogen is no exception. As you go higher up, atmospheric pressure drops. Since a liquid boils when its internal vapour pressure equals the external pressure, it takes less energy—and a lower temperature—to get it boiling at a higher elevation.
So, if you’re running a lab in the mountains, your liquid nitrogen will boil at a temperature slightly lower than the standard -195.8°C. While the difference might seem minor, it can be a critical detail when you’re calibrating sensitive scientific equipment that depends on exact temperature points for its accuracy.
At Cryonos GmbH, we build state-of-the-art cryogenic solutions engineered for ultimate safety and performance. Don't leave the integrity of your valuable samples to chance. Explore our complete range of storage and transport vessels at https://www.cryonos.shop and find the equipment you can trust.