No Products in the Cart
When you're working with liquid nitrogen, one of the first and most important numbers you need to know is its density. At its boiling point of -195.8°C, the standard density of liquid nitrogen (dichte stickstoff flüssig) is roughly 808 kilograms per cubic metre (kg/m³).
This isn't just a random statistic; it's the bedrock value for almost every calculation you'll make in cryogenics. Getting a solid grip on this number is the first step toward handling liquid nitrogen safely and efficiently.
So, what does that number actually mean in the real world? Density is simply a measure of how much "stuff" (mass) is packed into a given space (volume).
Let’s try a quick thought experiment. Picture a standard one-litre bottle of water. Now, imagine filling an identical bottle with liquid nitrogen. If you were to pick them both up, the liquid nitrogen bottle would feel surprisingly light. It would weigh only about 808 grams, while the water weighs a full 1,000 grams (or 1 kilogram).
This tells us something crucial: liquid nitrogen is about 20% less dense than water. This isn't just a fun fact for a trivia night; it has massive practical consequences for anyone handling cryogenic liquids.
Knowing the precise dichte stickstoff flüssig is non-negotiable. It’s the key to everything from calculating how much nitrogen is in a storage dewar to preventing serious safety hazards like overfilling or asphyxiation from gas expansion.
Why all the fuss over a single value? Because density is the bridge that connects volume—something that's easy to measure with a dipstick or level sensor—to mass, which is often what you really care about for inventory or safety. For engineers, lab technicians, and researchers, this number is fundamental. It impacts:
In short, the concept of dichte stickstoff flüssig is where nearly every practical cryogenic calculation begins. It turns abstract measurements into tangible, actionable data, forming the foundation of both operational efficiency and safety in any lab or industrial setting.
The density of liquid nitrogen isn't a fixed number. Think of it more as a fluid dance, with temperature calling the tune. As liquid nitrogen gets colder, its molecules slow down, lose energy, and pack together much more tightly.
Imagine a crowd of people on a chilly day. When the wind picks up, everyone instinctively huddles closer for warmth, making the group denser. Liquid nitrogen molecules behave in a similar way. This core relationship means that colder liquid nitrogen is always denser liquid nitrogen.
Grasping this principle is essential for anyone working with cryogenic liquids. Even a small shift in temperature can create a noticeable change in density, directly impacting things like storage calculations, transport weight, and overall process efficiency.
The graphic below shows the standard density values for liquid nitrogen right at its boiling point, presented in the most common units you'll encounter.
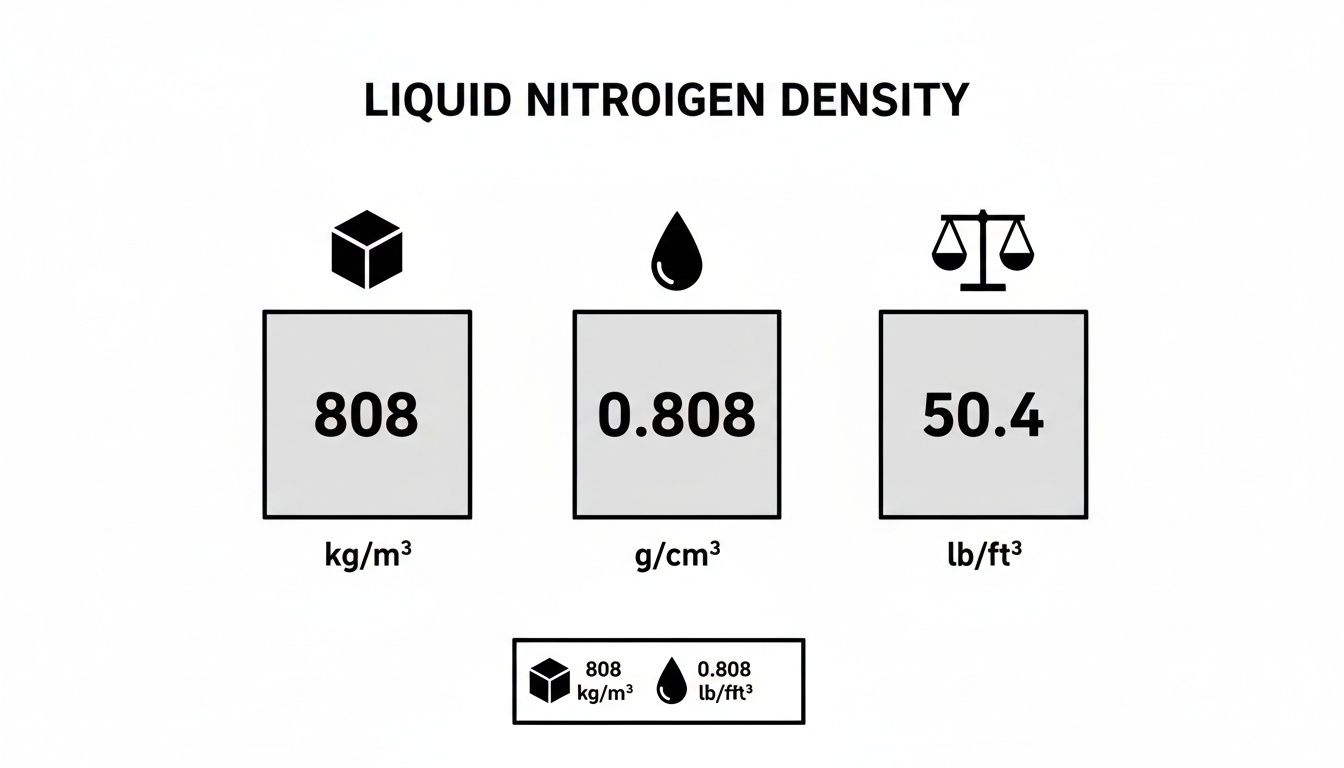
This confirms the key industry figure of 808 kg/m³ and gives you the direct conversions for other measurement systems used around the world.
When you chill liquid nitrogen below its normal boiling point of -195.8°C (77.3 K), it becomes what we call subcooled nitrogen. This is usually done by increasing the pressure or using specialised cooling systems. The main payoff? A significant bump in its density.
Cooling liquid nitrogen by just a few degrees, for example, makes it measurably denser. This might sound like a tiny detail, but for certain high-stakes industries, it’s a game-changer. In fields like rocket propulsion or high-tech manufacturing, every square centimetre of space is precious.
By using denser, subcooled nitrogen, engineers can store more mass of the liquid in the exact same volume. This maximises fuel loads for launch vehicles or boosts the cooling capacity for sensitive electronics without needing bigger tanks.
This ability to tweak density by controlling temperature is a powerful tool in cryogenics. You can dive deeper into how the temperature of liquid nitrogen impacts its properties in our detailed guide.
To see this principle in action, let's look at how the density changes as the liquid gets colder than its standard boiling point.
| Temperature (K) | Temperature (°C) | Density (kg/m³) |
|---|---|---|
| 77.3 | -195.8 | 808 |
| 75.0 | -198.1 | 820 |
| 70.0 | -203.1 | 843 |
| 65.0 | -208.1 | 865 |
As the table clearly shows, dropping the temperature from the boiling point of 77.3 K down to 65 K increases the density by over 7%. This seemingly small change has big consequences in precision applications.
The link between temperature and liquid nitrogen density isn't just a scientific curiosity; it's a practical reality for anyone managing cryogenic fluids. Here’s why it is so important:
Ultimately, treating density as a dynamic property allows for more precise control, better efficiency, and safer operations in any setting that relies on liquid nitrogen.
Now, let's bridge the gap between the physics of density and the day-to-day reality of handling cryogenic storage. Understanding the dichte stickstoff flüssig (density of liquid nitrogen) is the key to calculating precisely how much nitrogen, by mass, your storage vessel can actually hold. This is much more than a theoretical exercise; it's a vital calculation for smooth and safe operations.
Getting this right empowers you to manage your inventory effectively, prevent the serious risks of overfilling, and make sure you always have enough product on hand for your applications. It doesn't matter if you're working in biobanking, research, or industrial manufacturing—this calculation is fundamental.

Let's walk through a common, real-world example to see this principle in action. Imagine you have a standard 180-litre dewar that you need to fill. How many kilograms of liquid nitrogen will it contain once it's full?
The formula is refreshingly simple:
Mass (kg) = Density (kg/m³) x Volume (m³)
First things first, we need our units to match up. Our density is in kilograms per cubic metre (kg/m³), but our dewar's volume is in litres. Since there are 1,000 litres in one cubic metre, the conversion is straightforward.
Now we can plug this volume back into our main formula. We'll use the standard density of liquid nitrogen at its boiling point, which is 808 kg/m³.
And there you have it. Your 180-litre dewar holds approximately 145.44 kilograms of liquid nitrogen. This simple step turns a volume measurement into a precise mass, something absolutely essential for accurate inventory management and logistical planning. You can also dive deeper into the principles behind the density of a gas in our related article.
This formula isn't just for filling tanks; it’s a cornerstone of operational efficiency and safety in any cryogenic setting. Here’s how it gets applied:
Taking a physical property like density and turning it into a practical business tool is essential. This one calculation helps you manage resources, control costs, and maintain a seamless workflow, no matter the cryogenic application.
The need for this kind of precision is only growing. The global liquid nitrogen market was valued at USD 16.90 billion and is projected to reach USD 17.88 billion, largely driven by the chemical and pharmaceutical industries that absolutely depend on exact measurements. You can read more insights about the liquid nitrogen market growth on straitsresearch.com.
When you're working with cryogenic liquids, safety isn't just a priority—it's everything. The concept of liquid nitrogen's density might seem like a simple number, but in practice, it's one of the most critical tools we have for predicting and preventing hazards.
One of the most startling facts about liquid nitrogen is its massive expansion ratio. A single litre of liquid nitrogen will boil and expand into nearly 700 litres of nitrogen gas at room temperature. This isn't a gentle process; it's a rapid, forceful transformation that can have devastating consequences if not properly managed.

This is where understanding density becomes so important. If you know the volume of a liquid nitrogen spill, you can use its density to calculate the exact mass that's been released. From that mass, you can then figure out the enormous volume of gas it will turn into. This calculation is the cornerstone of any serious risk assessment.
Nitrogen gas itself isn't toxic, but it is a powerful asphyxiant. When it boils off, it pushes out the breathable air in a room. A significant spill, especially in a poorly ventilated space, can quickly drop the oxygen level from a safe 21% to dangerously low concentrations, leading to disorientation, loss of consciousness, or worse.
Calculating the potential volume of gas from a spill isn't optional—it's a non-negotiable step in safety planning. It’s what allows facility managers to design adequate ventilation systems, both passive and active, to ensure oxygen is replenished and nitrogen gas is safely vented.
This direct link between liquid mass and gas volume is fundamental to creating a safe working environment. It informs emergency response plans and helps shape the safe handling procedures that protect your team. You can dive deeper into the specific hazards of cryogenic liquids in our detailed safety guide.
The importance of density doesn't stop at the lab door. It's just as crucial for transport and logistics. Moving cryogenic liquids is a tightly regulated process, with strict rules governing vehicle weight and how loads are distributed.
Density calculations are essential for several reasons:
This connection between density and mass bridges the gap between the physics of cryogenics and the real-world protocols that keep people and property safe. In an industry where high-purity liquid nitrogen is key, safety must be equally precise. It's no surprise that cryogenic distillation is the leading production method, holding 42.6% of the market share, precisely because it delivers the purity and reliability that sensitive applications demand.
Working with liquid nitrogen across different industries, especially on a global scale, means you’ll inevitably run into different units of measurement. One technical sheet might list density in metric units, while a quote from an international partner uses imperial. This section is your straightforward toolkit for converting the dichte stickstoff flüssig (liquid nitrogen density) with confidence.
Knowing how to switch between these units is more than just a maths exercise; it’s about ensuring accuracy in every calculation, from storage capacity to transport weight. Below, we’ll walk through some simple formulas and clear examples to make these conversions second nature for anyone on your team.
The three most common units you'll see for liquid nitrogen density are kilograms per cubic metre (kg/m³), grams per cubic centimetre (g/cm³), and pounds per cubic foot (lb/ft³). Let's break down how to move between them without a hitch.
1. Kilograms per cubic metre (kg/m³) to Grams per cubic centimetre (g/cm³)
This is the simplest conversion of the bunch. Because there are 1,000 grams in a kilogram and 1,000,000 cubic centimetres in a cubic metre, the conversion factor is a clean 1,000. All you need to do is divide.
2. Kilograms per cubic metre (kg/m³) to Pounds per cubic foot (lb/ft³)
This one is absolutely essential when you're dealing with US or other imperial specifications. The key here is the conversion factor: one kilogram per cubic metre is roughly 0.06243 pounds per cubic foot.
By mastering these simple conversions, you eliminate the risk of costly errors that can pop up from inconsistent units. Whether you're ordering supplies, calculating shipping weights, or sizing a cryogenic vessel, getting the units right is crucial for both safety and efficiency.
These practical steps ensure that everyone on your team, no matter which measurement system they prefer, is working from the same accurate data. It removes ambiguity and makes for much smoother collaboration on projects that span different regions or follow different industry standards.
While you probably won't be out in the field measuring liquid nitrogen density every day, it’s worth understanding how it's done. The process reveals the incredible precision that goes into modern cryogenics. It’s not as simple as dropping a hydrometer into the liquid; the approach is more indirect but far more accurate.
For most day-to-day applications, direct measurement with specialised tools like cryogenic densitometers is overkill. These are expensive, complex instruments usually found only in laboratories or high-stakes industrial processes where absolute precision is non-negotiable.
Instead, the more common method relies on a smart combination of sophisticated sensors and established scientific data. This approach makes it clear that the dichte stickstoff flüssig (density of liquid nitrogen) isn't just some static value you pull from a textbook. It's a dynamic parameter, calculated in real-time.
Modern cryogenic management systems are designed to figure out the density by first measuring other key properties. Highly accurate temperature and pressure sensors are installed right inside the storage vessel, continuously monitoring the conditions of the liquid nitrogen.
These sensors feed live data into the system's control unit, and this is where the clever part happens. The system doesn’t actually try to measure density on its own. It cross-references the live data with established tables from globally recognised standards organisations, like the National Institute of Standards and Technology (NIST).
These tables contain exhaustive data, mapping out the exact density of nitrogen across a huge range of temperatures and pressures.
By comparing the live temperature and pressure readings from the sensors against this trusted data, the system can calculate the current density with exceptional accuracy. This method is both reliable and robust for industrial use.
This entire process highlights the advanced technology that underpins today's cryogenic systems. It ensures every calculation—from managing inventory levels to triggering safety protocols—is based on precise, real-time data rather than static assumptions. It’s the perfect blend of robust hardware and established scientific principles, turning complex physics into a practical and dependable tool for any operator.
Let's tackle some of the practical questions that pop up when you're working with liquid nitrogen. The goal here is to give you quick, clear answers that will help you in your day-to-day operations and troubleshoot any issues that might come up.
No, not even close. Liquid nitrogen is actually much lighter than water.
At its boiling point, liquid nitrogen has a density of about 808 kg/m³. Compare that to water, which sits at around 1,000 kg/m³. What this means in practice is that a one-litre container of liquid nitrogen is nearly 20% lighter than if you filled that same container with water.
This difference is absolutely critical when you're calculating transport weights and trying to predict how the liquid will behave in storage.
Yes, pressure is a major factor. When you increase the pressure on liquid nitrogen, you're essentially forcing the molecules closer together, which makes it denser.
This principle is exactly how subcooled nitrogen is created—a denser, colder state that's perfect for specialised applications where you need to pack more mass into the same volume. For most standard, open-to-atmosphere dewars, though, temperature is still the main driver of density changes.
The real art of cryogenics is in understanding how temperature and pressure work together. While temperature dictates most density changes in day-to-day use, pressure becomes the go-to control knob in high-performance or closed-loop systems.
It’s more the other way around. You can't directly measure density to determine the level, but you can measure the level to figure out the mass.
Technicians will typically use a dipstick or a dedicated level sensor to see how much liquid nitrogen is left in a dewar. This gives you the volume. From there, you just plug it into the density formula (Mass = Density x Volume) to calculate the exact mass in kilograms. It's the most reliable way to keep your inventory in check.
For state-of-the-art cryogenic solutions designed for precise and safe handling, trust Cryonos GmbH. Explore our comprehensive portfolio of storage vessels, transport units, and safety equipment at https://www.cryonos.shop.