No Products in the Cart
Yes, under specific conditions, nitrogen is dangerous. Although nitrogen gas makes up 78% of the air we breathe and is non-toxic, it comes with significant physical risks. The danger isn’t in the gas’s chemistry but in its physical properties: its ability to displace oxygen, its extreme cold as a liquid, and the immense pressure it creates when it turns back into a gas.
The best way to answer the question, "Is nitrogen dangerous?" is with an analogy. Water is essential for life, but it can be deadly as a flood or as scalding steam. Nitrogen is much the same. Its dangers depend entirely on its state (gas or liquid) and the environment it’s in. It's a silent risk because it’s colourless, odourless, and tasteless, meaning our senses can't warn us of impending danger.
There are three main hazards to be crystal clear about:
This diagram brings the three main hazards of nitrogen—asphyxiation, extreme cold, and pressure—into sharp focus.
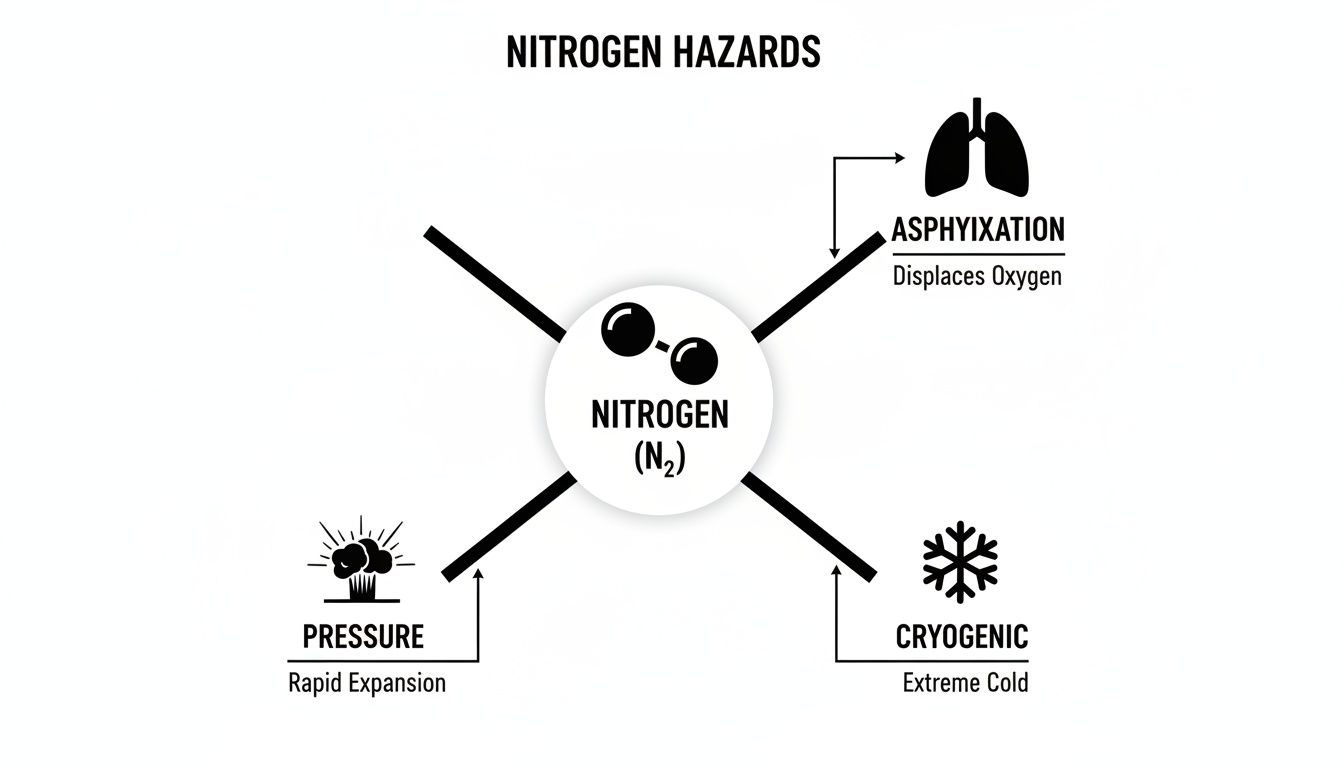
It’s a clear reminder that the risks are purely physical and that managing them comes down to solid, safe work practices.
To really understand the risks based on nitrogen's state, the table below offers a straightforward comparison. It helps you quickly grasp the specific dangers of gaseous versus liquid nitrogen and match them to the right safety measures.
| Hazard Type | Gaseous Nitrogen (GN2) | Liquid Nitrogen (LN2) |
|---|---|---|
| Asphyxiation | Primary Hazard. Displaces oxygen silently in enclosed spaces, leading to a high risk of suffocation. | High Risk. Boils off rapidly, creating large volumes of nitrogen gas that displace oxygen. |
| Cryogenic Burns | Not a risk. Gas is at ambient temperature. | Primary Hazard. Extreme cold (-196°C) causes severe tissue damage on contact. |
| Pressure/Explosion | Risk from high-pressure storage cylinders if mishandled or damaged. | High Risk. Rapid expansion upon vaporisation can rupture sealed containers with explosive force. |
This distinction is fundamental for creating robust safety protocols in labs, biobanks, and industrial facilities. Understanding these differences is the first step toward handling nitrogen safely, not fearfully.
The biggest danger with nitrogen is that it’s completely invisible to our senses. It has no colour, no odour, and no taste, which means we have absolutely no natural warning system to detect it when it starts pushing out the very oxygen we need to breathe. This makes asphyxiation—suffocation from a lack of oxygen—the most serious and deceptive risk you’ll face with gaseous nitrogen.
Think of it like pouring a heavy, invisible liquid onto the floor of a room. Nitrogen gas is slightly heavier than air, so it sinks and spreads out, pushing the lighter, breathable air up and away. It will quietly pool in low-lying areas, basements, pits, and other confined spaces, creating a deadly, oxygen-deficient trap.
Here’s the critical part: your body has no internal alarm for low oxygen. Unlike high levels of carbon dioxide, which make you feel an urgent need to gasp for air, a lack of oxygen doesn't trigger that same choking sensation. Instead, when you walk into a nitrogen-rich area, the first effects are subtle, almost gentle. You might feel a little light-headed, maybe even a bit euphoric.
This is what makes nitrogen so incredibly dangerous. There's no panic, no struggle. You can go from feeling slightly dizzy to completely unconscious in just a few seconds, without ever realising what’s happening. The progression is so fast and unforgiving that it leaves you no time to save yourself.
"The absence of sensory warning signs is the critical factor in nitrogen-induced asphyxiation. Victims often become incapacitated so quickly that they are physically unable to escape the hazardous environment, even if they momentarily recognise the danger."
Knowing what to look for is your only defence. As oxygen levels drop, your body and brain are starved, leading to a predictable—and rapid—decline in function.
This silent killer isn’t just a theoretical hazard; it has devastating real-world consequences. The German phrase "ist stickstoff gefährlich" (is nitrogen dangerous?) becomes tragically clear when you look at incidents involving any gas that displaces oxygen. Take the recreational misuse of nitrous oxide—"laughing gas"—as a parallel example.
In Germany, police-recorded incidents involving nitrous oxide shot up from just over 70 cases in 2021 to more than 300 in 2023. That’s more than a four-fold increase. Chillingly, 11 deaths were directly linked to nitrous oxide in 2023 alone, and experts believe the true number is even higher. These fatalities often happen when the gas displaces oxygen in a small, enclosed space like a car, leading directly to asphyxiation.
This stark example shows just how lethal asphyxiation risks are, whether it’s nitrogen in a lab or another gas in a different setting. For anyone working with these gases, it drives home the importance of safety protocols like inerting—a process you can explore in our guide on inerting with nitrogen. Mastering these practices is non-negotiable for countering the silent threat of oxygen displacement.
While the silent risk of asphyxiation is a primary concern with nitrogen gas, the liquid form—liquid nitrogen (LN2)—introduces an entirely different and immediate danger: extreme, damaging cold. Liquid nitrogen sits at a staggering -196°C (-320°F). To put that into perspective, it's a temperature so low that it can cause severe tissue damage far quicker than a hot flame.
Think about touching a white-hot piece of metal. Your body's reaction is instant—a severe thermal burn. Contact with liquid nitrogen is the cryogenic equivalent of that. The immense temperature difference between the LN2 and your skin causes cellular structures to freeze solid and rupture almost immediately. The result is a severe injury known as a cryogenic burn, or simply, frostbite.

This instantaneous freezing obliterates tissue on contact, making any direct exposure incredibly dangerous. Even a tiny splash can cause permanent damage, especially to sensitive areas like your eyes.
There’s a fascinating but treacherous phenomenon called the Leidenfrost effect that can create a false sense of security when working with LN2. When a small droplet of liquid nitrogen hits a much warmer surface (like your hand), it instantly vaporises, forming a thin, insulating layer of nitrogen gas. This vapour cushion can make LN2 droplets skitter and dance across a surface for a few seconds before they disappear.
This effect might trick you into thinking brief contact is harmless, but that's a deadly misconception. The protective vapour barrier is fleeting and completely unreliable. If the liquid gets trapped against your skin by clothing, or if the contact is more than a passing droplet, the intense cold will punch right through this barrier and cause a severe burn.
Relying on the Leidenfrost effect for safety is like assuming a thin sheet of ice will hold your weight—it's a gamble you simply can't afford to take. The protective barrier is temporary, and the consequences of it failing are both immediate and severe.
Because of this severe cryogenic hazard, handling liquid nitrogen without the right Personal Protective Equipment (PPE) is unthinkable. Your standard lab coat or a pair of latex gloves offer no real protection; the extreme cold will slice right through them in an instant. This is where specialised gear becomes mandatory.
Here’s the essential PPE you need to handle LN2 safely:
This equipment isn't just a list of recommendations; it’s the absolute minimum required to prevent life-altering injuries. Understanding just how cold liquid nitrogen truly is hammers home why these precautions are so critical.
The extreme cold of liquid nitrogen doesn't just harm living tissue—it can dramatically change the physical properties of many common materials. This process, known as cryogenic embrittlement, can lead to catastrophic equipment failure without warning.
When everyday materials like carbon steel, plastics, or rubber are exposed to -196°C, they lose their flexibility and become extremely brittle, almost like glass. A container made from the wrong type of plastic could shatter on contact with LN2, sending sharp fragments flying and releasing its hazardous contents. Likewise, a carbon steel pipe or fitting could fracture under the slightest stress.
This is precisely why only specific materials, such as stainless steel, copper, and certain aluminium alloys, are suitable for cryogenic applications. Using specialised cryogenic vessels, known as Dewars, is absolutely essential for safe storage and handling. These containers are engineered with a vacuum jacket to insulate the contents and are built from materials that can withstand the brutal temperature without becoming dangerously fragile. Ignoring these material constraints is a recipe for disaster.
While asphyxiation and cryogenic burns are often the first dangers that come to mind, the explosive potential of vaporising nitrogen is a hazard that’s far too often underestimated. The raw power behind this risk comes down to a startling fact of physics: one litre of liquid nitrogen expands to become nearly 700 litres of gas as it warms up.
To get a sense of this incredible expansion, picture a single drop of water instantly flashing into a massive cloud of steam. The force created by this rapid change from liquid to gas is immense. If this happens inside a sealed, non-vented container, the pressure builds at an unbelievable rate, quickly overwhelming the container's structural limits and causing it to fail catastrophically.

We're not talking about a slow leak here; this is a genuine explosion. The container fails violently, turning into shrapnel and releasing a huge cloud of nitrogen gas in an instant. This not only creates a blast hazard but also immediately introduces severe asphyxiation and cryogenic risks in the surrounding area.
Because this expansion is a fundamental property of nitrogen, any professionally engineered cryogenic equipment is built with this reality in mind. Specialised vessels like Dewars aren't just fancy thermos flasks; they are complex systems fitted with critical safety features to manage this constant pressure build-up.
These systems are designed to safely vent the expanding gas into the atmosphere, long before it reaches a dangerous level. They almost always include:
The question "ist stickstoff gefährlich" (is nitrogen dangerous?) finds a powerful answer right here. A properly vented container is perfectly safe; a sealed one is effectively a bomb waiting for a timer. These safety features aren't optional extras; they're non-negotiable engineering necessities.
Knowing how these pressure events happen in the real world is the absolute key to preventing them. The danger often comes not from some dramatic equipment failure, but from small, seemingly innocent oversights in the lab or during transport.
One of the most frequent—and dangerous—mistakes is improperly sealing cryogenic vials or sample tubes. If a standard, non-vented screw-cap tube is dipped into liquid nitrogen, a minuscule amount of the liquid can easily seep past the threads. When you pull that tube out and it starts warming to room temperature, that trapped LN2 instantly vaporises and expands. The resulting pressure can make the tube explode with surprising force, launching the cap and plastic fragments like a bullet.
Another insidious threat is the formation of ice plugs. If moisture from the air gets into the neck or vent path of a Dewar and freezes, it can form a solid plug that completely seals the container. Just like that, the vessel has become a closed system. The LN2 inside continues to evaporate, building pressure until the container itself fails violently. This is precisely why it’s so critical to keep equipment clean, dry, and regularly inspected.
These scenarios drive home a vital point: safe handling is everything. It’s about using professionally designed equipment and rigorously following protocols that prevent accidental sealing and contamination.
When a nitrogen-related emergency strikes, quick and correct action can be the difference between a close call and a tragedy. The real challenge is spotting the symptoms of exposure, which can be subtle and dangerously misleading. This section cuts through the theory to give you clear, practical steps for handling both nitrogen asphyxiation and cryogenic contact injuries.

Knowing how to respond is every bit as critical as understanding why the question "ist stickstoff gefährlich?" (is nitrogen dangerous?) is so important. Swift, informed action is what protects not only the victim but any potential rescuers from becoming casualties themselves.
Nitrogen asphyxiation is a silent killer, mainly because the person affected often has no idea they are in danger. The body doesn't have a built-in alarm for low oxygen, so the symptoms creep in and escalate rapidly as oxygen levels plummet, leaving a terrifyingly small window to react.
The progression is swift:
If you see someone collapse and suspect nitrogen exposure, your first instinct might be to rush in. Don't. Your own safety has to be the absolute priority.
Rescuer safety is paramount. Entering an oxygen-deficient atmosphere without Self-Contained Breathing Apparatus (SCBA) is a leading cause of multiple-fatality incidents. A would-be rescuer can become a victim in seconds.
Follow these emergency steps without fail:
Direct contact with liquid nitrogen causes a severe type of cold burn that needs specific, immediate first aid. Getting it wrong can make the tissue damage much, much worse.
When LN2 touches skin, the area will look pale or waxy-white and feel completely numb. As it thaws, the real trouble begins—it becomes intensely painful, swollen, and will likely blister.
Here’s the correct first aid protocol:
There are also a few critical things you must avoid doing at all costs:
Knowing the dangers is one thing, but preventing them is what really counts. Answering "ist stickstoff gefährlich?" with confidence means moving beyond theory and actively building a secure workspace. This isn't about a single solution; it's about a combination of solid engineering and smart, disciplined procedures. It's about making sure your physical space and your team are ready for the risks.
A truly safe facility doesn't just hang its hat on one safety measure. Instead, it layers multiple lines of defence, from the building's fundamental design right down to the daily habits of its staff. It's about weaving safety into the fabric of every action.
The best safety measures are the ones you don't have to think about—they're built right into the environment. These engineering controls are designed to automatically deal with hazards, taking human error out of the equation as much as possible. When you're working with nitrogen, two controls are completely non-negotiable: proper ventilation and continuous monitoring.
Good ventilation is absolutely critical. Since nitrogen gas is a bit heavier than air and totally invisible, it can silently pool in low-lying spots, pits, or any room without good circulation. A well-designed ventilation system is your active defence, constantly pulling this displaced air out and pushing fresh, oxygen-rich air in. This is what stops those deadly, oxygen-starved pockets from forming.
But ventilation can't do the job alone. You need to know the instant something goes wrong. That's where oxygen monitoring systems come in.
Think of an oxygen monitor as your sixth sense, because it's the only one that can detect a low-oxygen environment. Humans simply cannot feel oxygen levels dropping. These alarms are your only warning before your judgment becomes impaired and you pass out.
These aren't just plug-and-play gadgets; their placement and setup are vital for them to be effective.
If engineering controls manage the physical space, administrative controls manage the people working in it. These are your rules, procedures, and training programs—the human element of your safety system. They ensure everyone understands the risks and knows exactly how to work safely.
The bedrock of good administrative control is a set of clear Standard Operating Procedures (SOPs). These need to be detailed, step-by-step guides for every single task involving nitrogen, from something as simple as connecting a cylinder to filling a large cryogenic vessel. For a great starting point, check out these 7 important rules for safe work with cryogenic liquids.
Beyond having written rules, you need comprehensive training. Every single person who works with or even near nitrogen has to be properly educated on its unique hazards. This training must cover how to recognise the symptoms of asphyxiation and cold burns, what to do in an emergency, and how to correctly use all required PPE. This isn't a one-time thing, either—regular refresher courses are crucial to keep this knowledge front and centre.
The silent, creeping nature of airborne hazards is a powerful reminder of why these protocols matter so much. Take nitrogen dioxide (NO2), for instance. A 2022 analysis in Germany found a statistical link between long-term NO2 exposure and more severe COVID-19 outcomes. For every 1 µg/m³ increase in ambient NO2, the need for ICU beds climbed by 4.2% during the first wave. This shows how even related nitrogen compounds can create unseen health risks, driving home the need for strict environmental and procedural controls. You can read more on this in the 2022 study on nitrogen dioxide and public health.
Finally, a simple but incredibly effective measure is to implement a buddy system for any high-risk task. This ensures no one ever works alone in a potentially dangerous situation. It gives you an immediate second pair of eyes to spot trouble and a partner ready to help in an emergency.
When you work with nitrogen day-in and day-out, practical questions are bound to come up. This is where theory meets reality. Below, we've tackled some of the most common queries we hear, providing clear, direct answers to help you turn safety knowledge into confident action on the ground.
While every piece of your Personal Protective Equipment (PPE) plays a role, the full face shield and cryogenic gloves are absolutely critical. Think of it this way: your eyes are incredibly vulnerable, and even a minuscule splash of LN2 can cause permanent, life-altering damage. A full face shield is non-negotiable.
Your hands are next in line. Cryogenic gloves are specially insulated to shield you from the severe burns that come from accidental contact. One crucial tip from the field: always wear your gloves loosely. If any liquid nitrogen ever splashes inside a glove, you need to be able to fling it off instantly before the liquid gets trapped against your skin.
Absolutely not. This is one of the most dangerous mistakes anyone can make, and it should never, ever be attempted. Transporting liquid nitrogen in an enclosed personal vehicle is a recipe for disaster.
Even a small spill will instantly vaporise, expanding to displace the oxygen inside your car's cabin in a matter of moments.
This creates a silent, invisible, and fatal asphyxiation hazard. The driver and any passengers can lose consciousness and perish without any warning signs like choking or gasping for air.
Professional transport requires specially ventilated vehicles and certified containers designed for the job. The German question, "ist stickstoff gefährlich?" (is nitrogen dangerous?), becomes a life-or-death reality in this exact scenario. The risk is simply too great.
Here's the scary part: you don't. Humans have no natural ability to sense a lack of oxygen. There’s no smell, no taste, and you won’t feel a choking sensation. By the time you feel dizzy or confused, your cognitive functions are already severely impaired, and you may be physically unable to escape.
This is precisely what makes nitrogen asphyxiation so insidious. The only reliable way to know if a space is safe is to use a calibrated oxygen monitoring system. These devices are your lifeline. They will sound a loud, unmistakable alarm—usually when oxygen levels dip below the safe threshold of 19.5%—giving you the critical warning you need to evacuate immediately.
For state-of-the-art cryogenic solutions that prioritise safety and reliability, trust Cryonos GmbH. Explore our comprehensive portfolio of storage vessels, transport units, and safety equipment. Visit us at https://www.cryonos.shop to find the right solution for your needs.