No Products in the Cart
At what temperature does oxygen turn into a liquid? The quick answer is -183°C (-297.4°F or 90.15 K), but that's just the beginning of the story.
This isn't just a number; it's the boiling point of liquid oxygen at standard atmospheric pressure. It’s the critical threshold where this incredibly useful, pale blue liquid starts to vigorously transform back into an invisible gas. Getting a real feel for this temperature is key to handling liquid oxygen safely and using it effectively in any setting, from laboratories to large-scale industrial sites.
Liquid oxygen, often called LOX, is more than just "cold." It exists in a state of extreme cold that's hard to wrap your head around. To put it in perspective, the difference between a freezing day (0°C) and the boiling point of liquid oxygen is a staggering 183 degrees. This isn't just chilly; it's a profoundly different thermal environment, one that makes LOX a powerful tool but also a serious hazard if you don't respect it.
That precise boiling point of -183°C isn't arbitrary. It’s the exact temperature where, at normal sea-level pressure, oxygen molecules absorb enough energy from their surroundings to break free from their liquid bonds and escape as a gas. The process of creating LOX involves chilling gaseous oxygen down to this point, a cornerstone of many cryogenic industrial applications.
You can see this transformation happening in real-time in the image below.
What you're seeing is the liquid oxygen violently boiling away. To the LOX, the warmth of a normal room is like putting a pot of water on a blazing stove—it provides a massive amount of energy, causing it to rapidly vaporise.
Working in cryogenics means you'll constantly jump between different temperature scales. Whether you're a scientist, an engineer, or a technician, knowing how to translate -183°C into Fahrenheit or Kelvin is a must-have skill.
The Kelvin scale is the go-to for scientists in this field. It's an absolute scale, meaning 0 K is absolute zero—the theoretical point where all molecular motion grinds to a halt. This makes it perfect for the precise calculations needed in cryogenics.
Here’s a simple table to make those conversions quick and painless.
| Unit | Temperature |
|---|---|
| Celsius | -183°C |
| Fahrenheit | -297.4°F |
| Kelvin | 90.15 K |
Keep this table handy. It ensures everyone on the team, regardless of their background, is speaking the same language when it comes to temperature.
One of the biggest mistakes you can make is assuming that the -183°C boiling point is a fixed, universal constant. It’s not. In the real world, this temperature is completely tied to the surrounding pressure. It's a fundamental dance of physics that works like this:
This principle is absolutely vital for anyone designing or using cryogenic storage tanks and transport vessels. These containers are often slightly pressurised to strategically raise the boiling point, which helps manage the inevitable "boil-off" and keeps the oxygen in its liquid state for as long as possible.
Knowing the boiling point of liquid oxygen is one thing, but truly understanding how to work with it safely means getting to know its unique personality. You have to grasp the fundamental properties that dictate how it behaves under different conditions, and these characteristics go far beyond a single temperature reading.
Think of these properties as the unwritten rules for handling liquid oxygen. Ignoring them is like trying to drive without knowing what a stop sign means. Terms like density, viscosity, and specific heat aren't just for textbooks; they have direct, practical consequences for everything from storage and transport to equipment design.
This quick visual guide breaks down the boiling point of liquid oxygen across the three main temperature scales you'll encounter in scientific and industrial work.
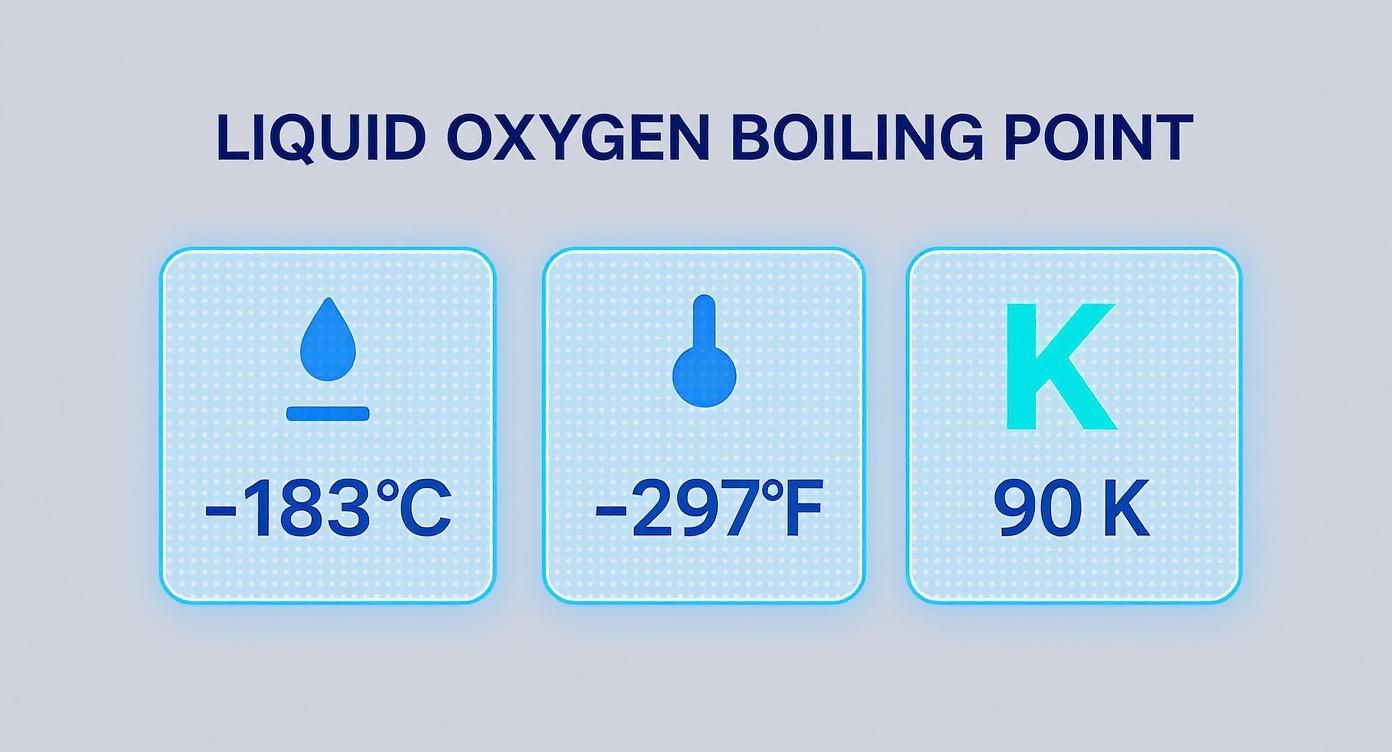
It really puts into perspective the extreme cold needed to keep oxygen in its liquid state—a critical fact for anyone handling cryogenic materials.
One of the best tools for understanding how a substance acts is its phase diagram. You can think of it as a complete "state map" for oxygen, showing you exactly which combinations of temperature and pressure will cause it to exist as a solid, a liquid, or a gas.
The lines on this map, called phase boundaries, are what really matter. The line separating the liquid and gas phases, for example, traces the boiling point at different pressures. Follow that line, and you can see precisely how the boiling temperature shifts as pressure goes up or down.
Beyond the phase diagram, a few other properties are vital for day-to-day applications. Each one tells a part of the story of how liquid oxygen will behave when you're actually using it.
Here are some of the most important characteristics to get your head around:
Key Takeaway: The physical properties of liquid oxygen are all interconnected. A small change in temperature doesn't just bring it closer to boiling; it also changes its density, which affects how it's stored and measured. A deeper look into the properties of oxygen can give you even more insight.
Understanding these properties is far from a theoretical exercise. It directly shapes the engineering and safety protocols that are non-negotiable when handling liquid oxygen.
For instance, the extreme cold dictates the choice of materials for pipes and containers. Many common materials, like carbon steel, become incredibly brittle and can shatter on contact with LOX. That's why engineers have to use specialised materials like stainless steel or certain aluminium alloys that stay ductile even at brutally low temperatures.
Even more critical is the expansion ratio of oxygen from liquid to gas—it's a massive 860 to 1 at standard conditions. That means one litre of liquid oxygen will expand to fill about 860 litres of space as a gas. This single property is why proper ventilation and pressure-relief systems on storage tanks are absolutely essential. Without them, the pressure from boil-off could lead to a catastrophic container failure.
Measuring the brutal cold of liquid oxygen isn't a job for your everyday thermometer. Trying to use a standard mercury or alcohol thermometer is like attempting to measure a single hair with a metre stick—it’s the wrong tool for the job and will fail spectacularly.
Before they even get close to the -183°C boiling point of liquid oxygen, the liquids inside these common thermometers will freeze solid. This renders them completely useless. For accurate, reliable data in cryogenic conditions, you need specialised instruments built to handle the extreme cold. Professionals in labs and industrial settings typically rely on two main types of sensors: resistance thermometers and thermocouples.
The gold standard for this kind of work is the Platinum Resistance Thermometer (PRT), with the Pt100 model being a particularly popular choice. The principle behind it is beautifully simple and predictable: as the temperature plummets, the electrical resistance of the platinum wire inside drops in a stable, well-documented way. By measuring this resistance, you can calculate the temperature with incredible precision.
Another workhorse is the thermocouple, especially types like the Type T (copper-constantan) or Type K. These function by measuring the tiny voltage generated when two dissimilar metals are joined at points with different temperatures. While they are generally less accurate than PRTs, thermocouples are tough, affordable, and work across a massive temperature range, making them a great fit for many industrial monitoring tasks.
A critical point to remember is calibration. For any scientific research or process-critical application, using a sensor that has been specifically calibrated for cryogenic temperatures is non-negotiable. This ensures your readings aren't just consistent, but truly accurate.
Having the right sensor is only half the battle. How and where you place it is just as crucial. Small slip-ups in your technique can introduce surprisingly large errors, leading to bad data and even potential safety hazards.
To make sure your measurements are spot on, stick to these essential guidelines:
This image shows a typical industrial-grade Pt100 resistance probe, a go-to tool for cryogenic temperature measurement.
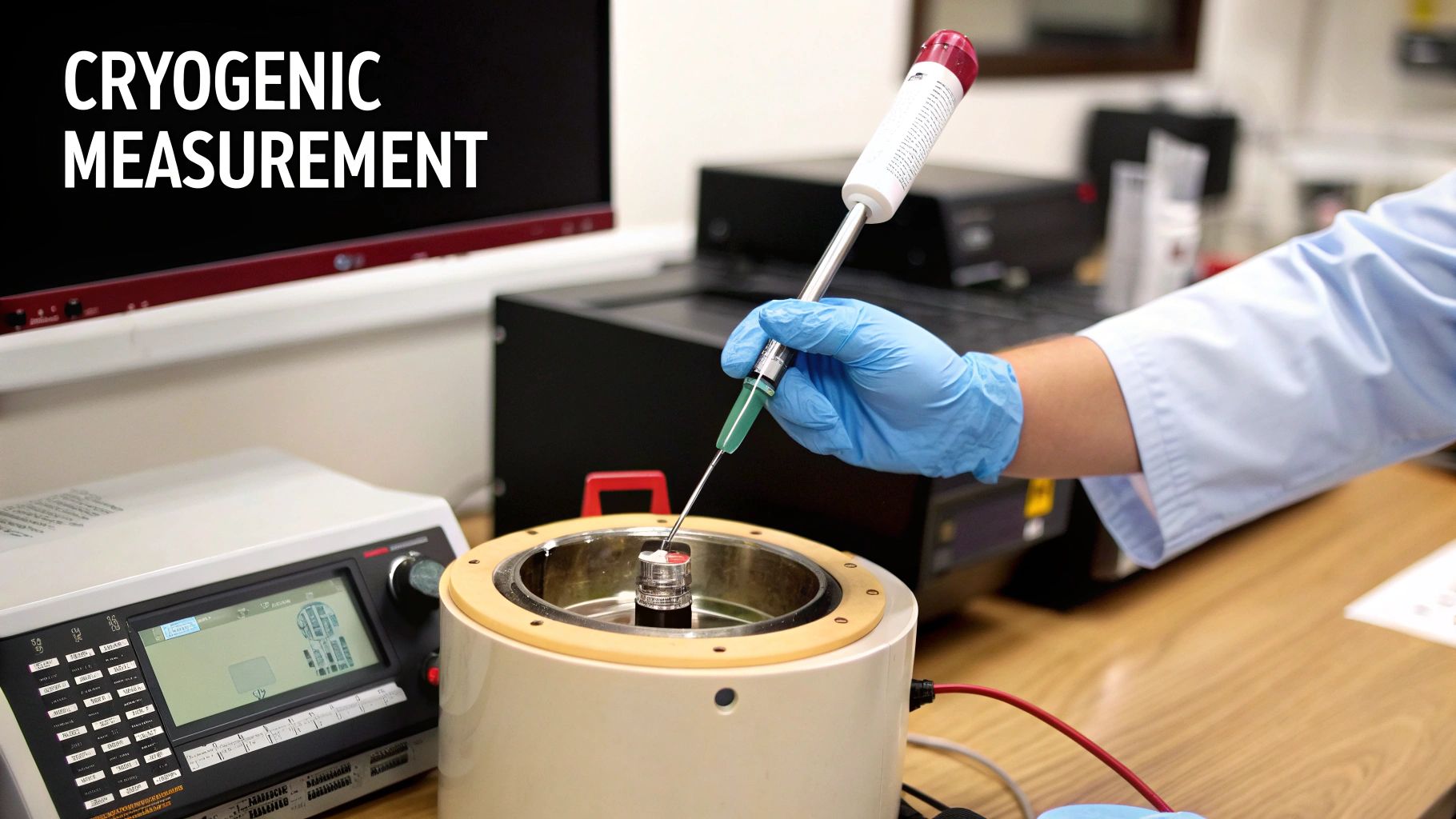
You can see the robust metal sheath and secure connector, both designed to withstand the tough conditions of industrial and lab settings while delivering a reliable signal. By mastering these tools and techniques, you can confidently and accurately measure the temperatur flüssiger sauerstoff.
Containing a substance as intensely cold as liquid oxygen presents a remarkable engineering challenge. The main goal is simple but incredibly difficult: keep the heat out. Any warmth that manages to sneak into a storage container will cause the LOX to boil, turning the precious liquid back into gas. This constant evaporation is known in the industry as boil-off.
To fight this ever-present enemy, engineers have developed sophisticated cryogenic containers that are essentially high-tech thermos flasks. Whether it's a small lab-sized Dewar or a massive industrial tank, the design principle is the same: a double-walled construction is key.
The real magic happens in the space between those two walls. A near-perfect vacuum is pulled in this gap, which dramatically cuts down on heat transfer through both convection and conduction. On top of that, the inner surfaces are often coated with highly reflective materials that act like mirrors, bouncing thermal radiation away before it can ever reach the liquid oxygen inside.
Choosing the right container for liquid oxygen really comes down to the job at hand. You have to balance how much you need, whether it needs to be portable, and how long you need to store it. Each type of container is specifically engineered to minimise heat getting in and to manage the pressure that inevitably builds from boil-off.
A comparative overview of different LOX storage solutions, highlighting their capacity, applications, and key features.
| Container Type | Typical Capacity | Primary Use Case | Key Feature |
|---|---|---|---|
| Dewar Flasks | 1 - 50 Litres | Laboratories, small-scale medical use | Unpressurised with loose-fitting lid |
| Liquid Cylinders | 80 - 240 Litres | Workshops, hospitals, small industrial | Pressurised for gas or liquid withdrawal |
| Microbulk Tanks | 1,000 - 5,000 Litres | Continuous supply for moderate users | On-site refilling from a delivery truck |
| Bulk Storage Tanks | > 10,000 Litres | Production facilities, large industry | Massive capacity for major operations |
Each of these containers fills a specific niche, from benchtop lab work to fuelling rockets, but all are built around the core principles of vacuum insulation to keep the cryogenic liquid safe and stable.
In Germany and across Europe, large-scale vertical tank storage is the most common technology, making up the largest market segment thanks to its efficiency in big industrial settings. When stored correctly in cool, dry places away from direct sunlight, liquid oxygen has a shelf life of up to 60 months (5 years). This makes it a viable option for strategic reserves and long-term industrial planning.
No matter the size of the container, every cryogenic vessel must be equipped with robust safety features to handle the constant pressure build-up from the vaporising liquid. The most important of these is the pressure relief valve.
This device is a non-negotiable safety component. As heat slowly leaks in and boils the LOX, the resulting oxygen gas expands, causing the internal pressure to climb. The relief valve is designed to automatically pop open at a preset pressure, safely venting the excess gas to prevent a dangerous and potentially catastrophic tank rupture.
Think of a pressure relief valve as the container's essential safety net. It's the mechanism that ensures the immense expansion from liquid to gas—a ratio of nearly 860:1—is managed safely, preventing the vessel from turning into a pressurised bomb.
Getting liquid oxygen from a production plant to a hospital, lab, or factory involves another layer of specialised logistics and very strict rules. LOX is moved around in custom-designed tanker trucks, which are essentially large, mobile Dewar flasks built to handle the bumps and vibrations of the road.
These tankers use the same vacuum insulation and pressure relief systems you'd find on stationary tanks. They also have internal walls, called baffles, that stop the liquid from sloshing around too much during transit, which keeps the truck stable.
Moving materials like this is governed by stringent regulations, like the ADR (European Agreement concerning the International Carriage of Dangerous Goods by Road). These rules dictate everything from how the vehicle is built and how the driver is trained to the specific labels and paperwork required for every single trip. For anyone involved in logistics, understanding the regulations for transporting gas cylinders is not just good practice—it's the law.
Let's be clear: working with liquid oxygen isn't like handling most other industrial substances. The extreme temperatures involved introduce a unique set of hazards that demand your full respect and a strict, unwavering commitment to safety protocols. One slip-up can lead to horrific injuries or catastrophic equipment failure.
Whether you're in a lab, a biobank, or an industrial facility, understanding and managing these risks is non-negotiable.

The dangers really boil down to three main categories. Get these right, and you'll create a secure environment for yourself and your team.
The most immediate and obvious danger of liquid oxygen is its bone-chilling cold. Even a fleeting moment of direct skin contact will cause severe cryogenic burns—which are, for all practical purposes, identical to thermal burns and can destroy tissue in an instant. Even the cold gas boiling off the liquid is a threat.
This is why a complete set of cryogenic Personal Protective Equipment (PPE) is mandatory. There are no shortcuts here.
Always make sure your PPE is dry and fits loosely. Your trousers should be worn outside of your boots—this prevents any spilled liquid from getting trapped against your skin.
Beyond personal safety, the intense cold of liquid oxygen is a serious threat to the equipment and materials around it. Many common materials that are strong and pliable at room temperature become something else entirely when exposed to -183°C.
This phenomenon is called cryogenic embrittlement, or brittle fracture. Imagine a stretchy rubber band. If you flash-froze it with LOX, it would become hard and fragile, shattering like glass if you tried to bend it. The same thing happens to certain metals.
For instance, carbon steel—a common construction material—loses all its ductility at cryogenic temperatures and can fracture suddenly under stress. This is why every single piece of equipment intended for LOX service, from pipes and valves to storage containers, must be made from compatible materials.
Materials suitable for cryogenic service include:
Using the wrong material isn’t a question of if it will fail, but when. Always double-check that every component in your system is rated for cryogenic use.
The final major hazard is maybe the most deceptive because it's invisible. As liquid oxygen evaporates, it releases massive volumes of pure oxygen gas, dramatically increasing the oxygen concentration in the air. Normal air has about 21% oxygen, but a spill or leak can quickly create an oxygen-enriched atmosphere.
This environment won't just spontaneously combust, but it makes everything hyper-flammable. Materials that you wouldn't think of as flammable, like safety clothing, can ignite with ease and burn with terrifying speed. Think of it like fanning a tiny flame—the extra oxygen turns a simple spark into an inferno.
Because of this hidden danger, it's absolutely critical to:
Even with the best training, things can get weird when you're working with something as extreme as liquid oxygen. Strange noises, unexpected frost—these are the real-world situations that pop up in labs, biobanks, and industrial settings. This is your go-to guide for those moments when you need a quick, reliable answer.
Think of these common issues as warning signs. Knowing how to read them can be the difference between catching a small problem and dealing with a major incident. Let's get into what you might encounter.
If you see a thick layer of frost or ice building up on the outside of a cryogenic container, that’s a major red flag. This isn't just normal condensation from a cold surface; it's a clear sign that the vacuum insulation between the container's inner and outer walls has failed.
That vacuum is the tank’s main line of defence against heat. When it's gone, the super-cold inner wall starts flash-freezing moisture right out of the air and onto the outer surface.
A compromised vacuum isn't just an efficiency problem—it's a safety hazard. The liquid oxygen will boil off much faster than the system can handle, causing a rapid pressure spike. If you see this happening, that container needs to be taken out of service immediately and looked at by a professional.
This is a serious failure. The vessel has lost its ability to insulate properly and may need to be decommissioned to prevent any further risk.
A little bit of boil-off is normal for any cryogenic container. It's the natural, slow evaporation that happens as tiny amounts of heat inevitably find their way in. But if you suddenly notice that you're losing a lot more product than usual, that’s your system telling you something is wrong.
Think of a high boil-off rate as a fever for your cryogenic tank—it’s a symptom of a deeper problem. It means heat is getting into your liquid oxygen much, much faster than it should be.
Here are the usual suspects:
A high boil-off rate is more than just a waste of money; it's a clear maintenance alert that your system needs a thorough check-up.
Seeing a pool of that intensely cold liquid can be unnerving, but small spills are manageable if you follow the right steps. Your absolute top priority is ventilation. You have to prevent an oxygen-enriched atmosphere from building up, as this creates a serious fire hazard.
For a minor spill in an open or well-ventilated area, the best response is usually the simplest one: let it evaporate on its own. Your job is to clear the area of people and remove anything that could possibly create a spark.
Here are the critical "dos and don'ts" for a small LOX spill:
Do:
Don't:
The key here is to respect the physics of liquid oxygen—its intense cold and its massive expansion as it turns back into a gas. Give it space and air, and let it do its thing safely.
For state-of-the-art cryogenic solutions designed for maximum safety and efficiency, trust the experts at Cryonos GmbH. Explore our complete range of storage vessels, transport units, and safety equipment at https://www.cryonos.shop.